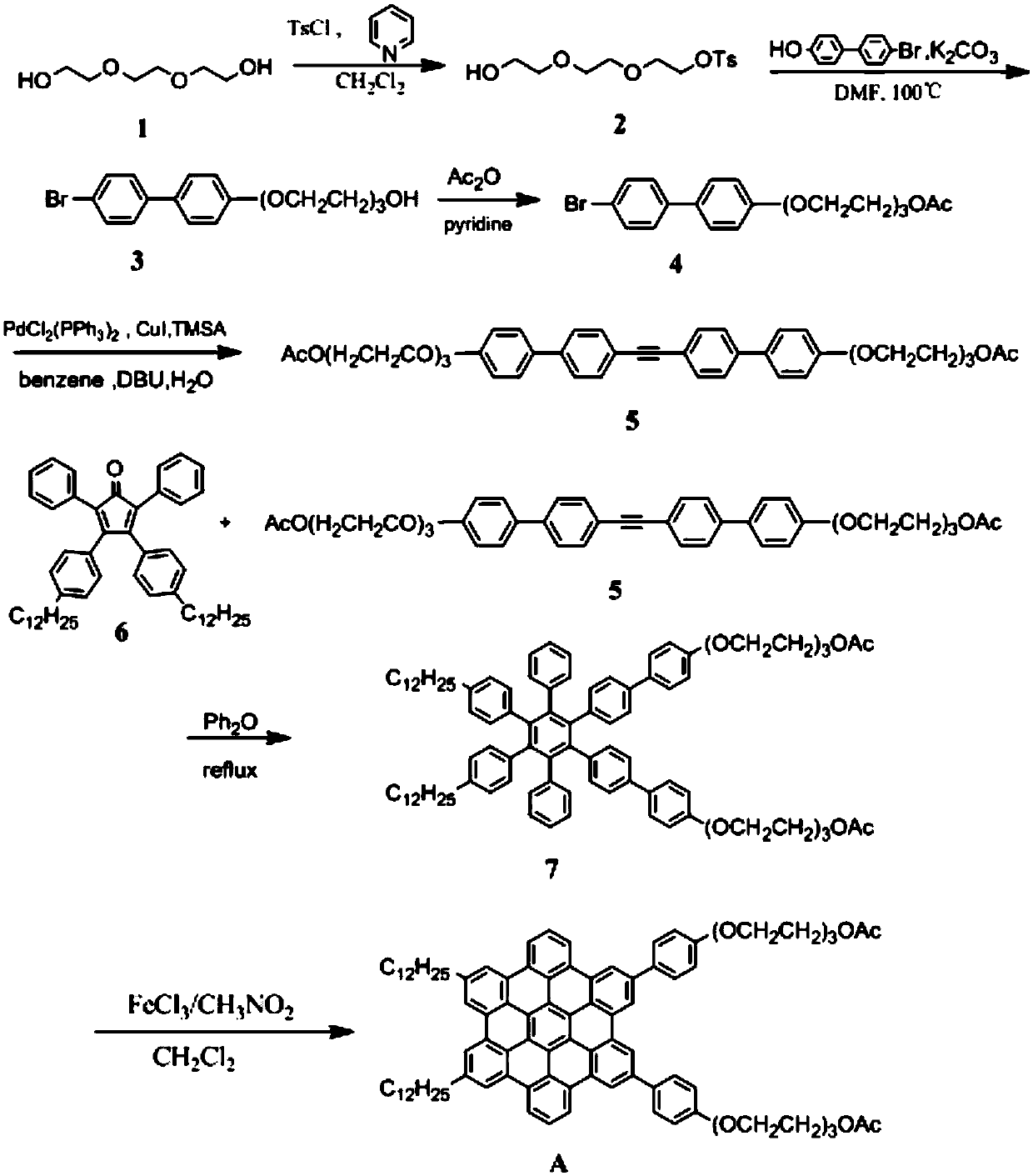
Preparation method of organic graphene nano-tubes with titanium dioxide modified on the surface
A titanium dioxide, surface modification technology, applied in nanotechnology, chemical instruments and methods, nanotechnology and other directions for materials and surface science, to achieve the effect of strong stability and enhanced energy transmission efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
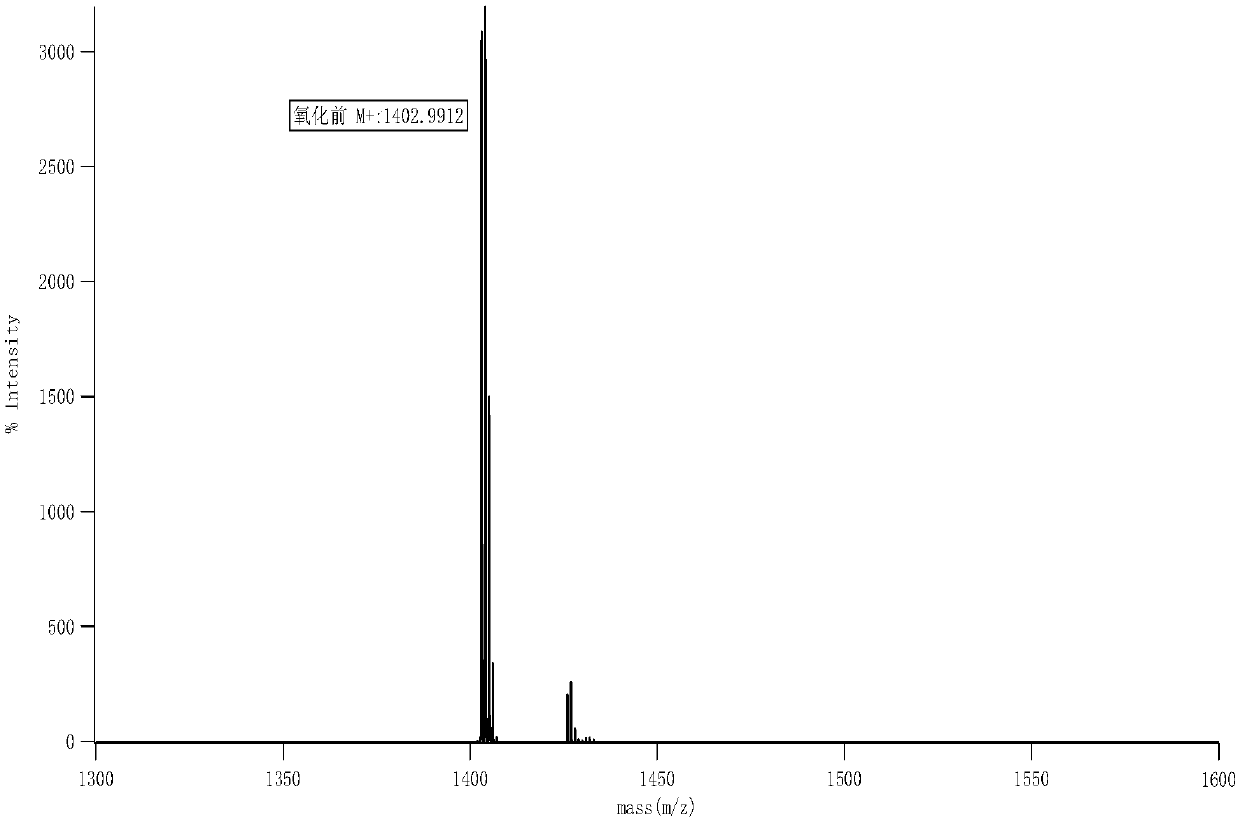
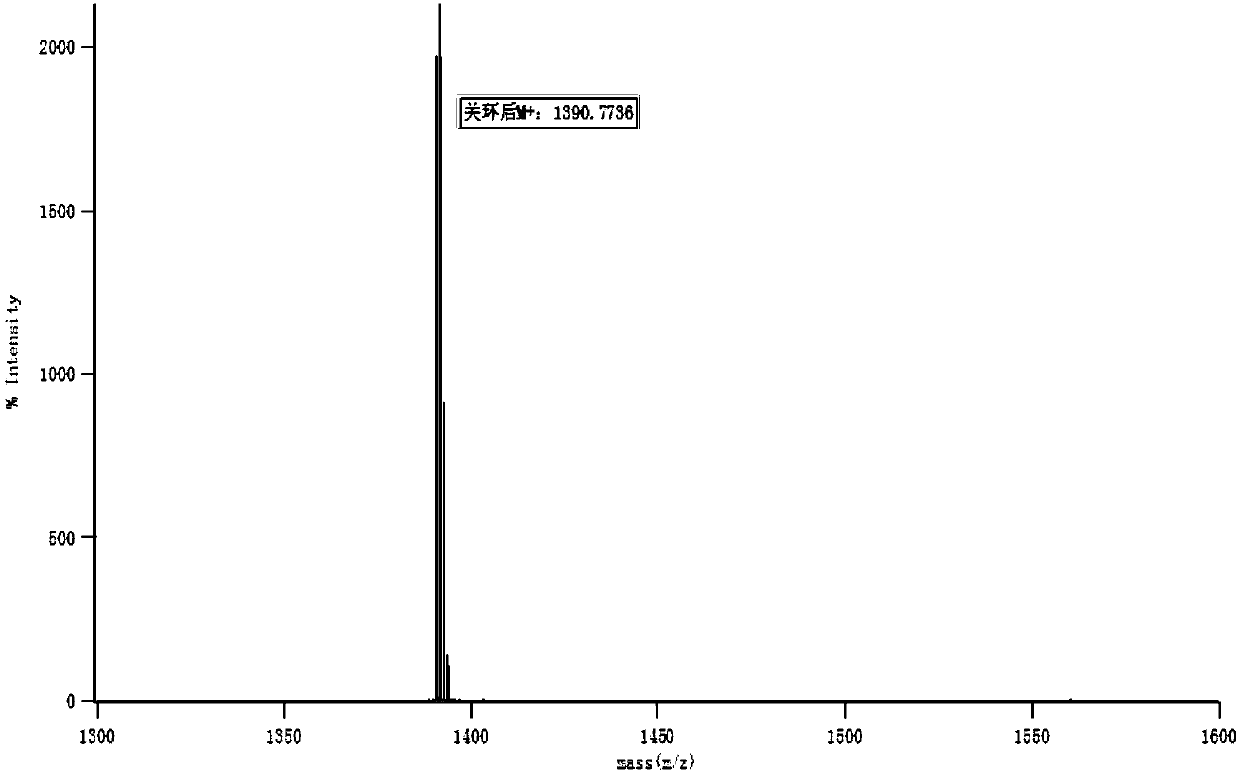
Image
Examples
Embodiment 1
[0041] Preparation of compound 2
[0042] Add triethylene glycol (2g, 13.3mmol, 1eq) into a 250mL two-necked flask, dissolve it in 20mL THF, pump for ventilation, protect with Ar, and slowly add 1.5mL of pyridine dropwise at 0°C. TsCl (3.01g, 15.84mmol, 1.3eq) was dissolved in 50mLTHF, and the reaction solution was slowly added dropwise, and stirred overnight. After acidification, the reaction solution was extracted with DCM, then washed with saturated NaCl, and most of the solvent was evaporated by rotary evaporation, and an appropriate amount of silica gel was added for dry column chromatography and eluent (EA) to obtain 4 g of white liquid, namely compound 2, the yield 98%.
[0043] 1 H NMR (400MHz, CDCl 3 )δ7.78(d,J=8.3Hz,2H),7.33(d,J=8.1Hz,2H),4.17–4.12(m,2H),3.68(dt,J=4.6,3.8Hz,4H), 3.60–3.52(m,6H),2.60(s,1H),2.43(s,3H).
[0044] MS-EI (C 13 h 20 o 6 S) Theoretical value [M] + : m / z 304.10. Measured value: 304.6.
Embodiment 2
[0046] Preparation of compound 3
[0047] Compound 2 (800mg, 2.63mmol, 1eq), p-bromobiphenol (1.63g, 6.58mmol, 2.5eq), K 2 CO 3 (3.63g, 26.3mmol, 10eq), 25mL of dimethylformamide, and gas exchange three times under the protection of Ar. After reacting at 100°C for 24 hours, add p-bromobiphenol, dimethylformamide and a small amount of 18-crown ether, and react for another 24 hours. Spin to dry, extract with dichloromethane, wash with water three times, dry over anhydrous magnesium sulfate, and elute with dichloromethane and ethanol sequentially after column chromatography to obtain 600 mg of white solid, namely compound 3, with a yield of 65%.
[0048] 1 H NMR (400MHz, CDCl 3 )δ7.52(d, J=8.0Hz, 2H), 7.47(d, J=8.2Hz, 2H), 7.40(d, J=8.0Hz, 2H), 6.98(d, J=8.2Hz, 2H) ,4.18(t,J=4.6Hz,2H),3.89(t,J=4.6Hz,2H),3.77–3.69(m,6H),3.65–3.59(m,2H).
[0049] MS-EI (C 18 h 21 BrO 4 ) theoretical value [M] + : m / z 380.06. Measured value: 381.2.
Embodiment 3
[0051] Preparation of Compound 4
[0052] After adding compound 3 (600mg, 1.58mmol, 1eq) and acetic anhydride (217mg, 2.25mmol, 1.5eq) into a 50mL two-neck flask, dichloromethane was added to dissolve it, and the gas was exchanged three times under the protection of Ar. Pyridine (0.2mL, 1.5eq) was added at 0°C, and after 20min, it was moved to room temperature and reacted overnight. After raising the temperature to 60°C and refluxing for 3 hours, it was washed three times with saturated ammonium chloride aqueous solution, dried over anhydrous magnesium sulfate, and spin-dried. After column chromatography, dichloromethane was eluted to obtain 530 mg of white solid, that is, compound 4, and the yield was 80%.
[0053] 1 H NMR (400MHz, CDCl 3 )δ7.52(d, J=8.0Hz, 2H), 7.47(d, J=8.2Hz, 2H), 7.40(d, J=8.0Hz, 2H), 6.98(d, J=8.2Hz, 2H) ,4.18(t,J=4.6Hz,2H),3.89(t,J=4.6Hz,2H),3.77–3.69(m,6H),3.65–3.59(m,2H).
[0054] MS-EI (C 20 h 23 BrO 5 ) theoretical value [M] + : m / z 423.30....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com