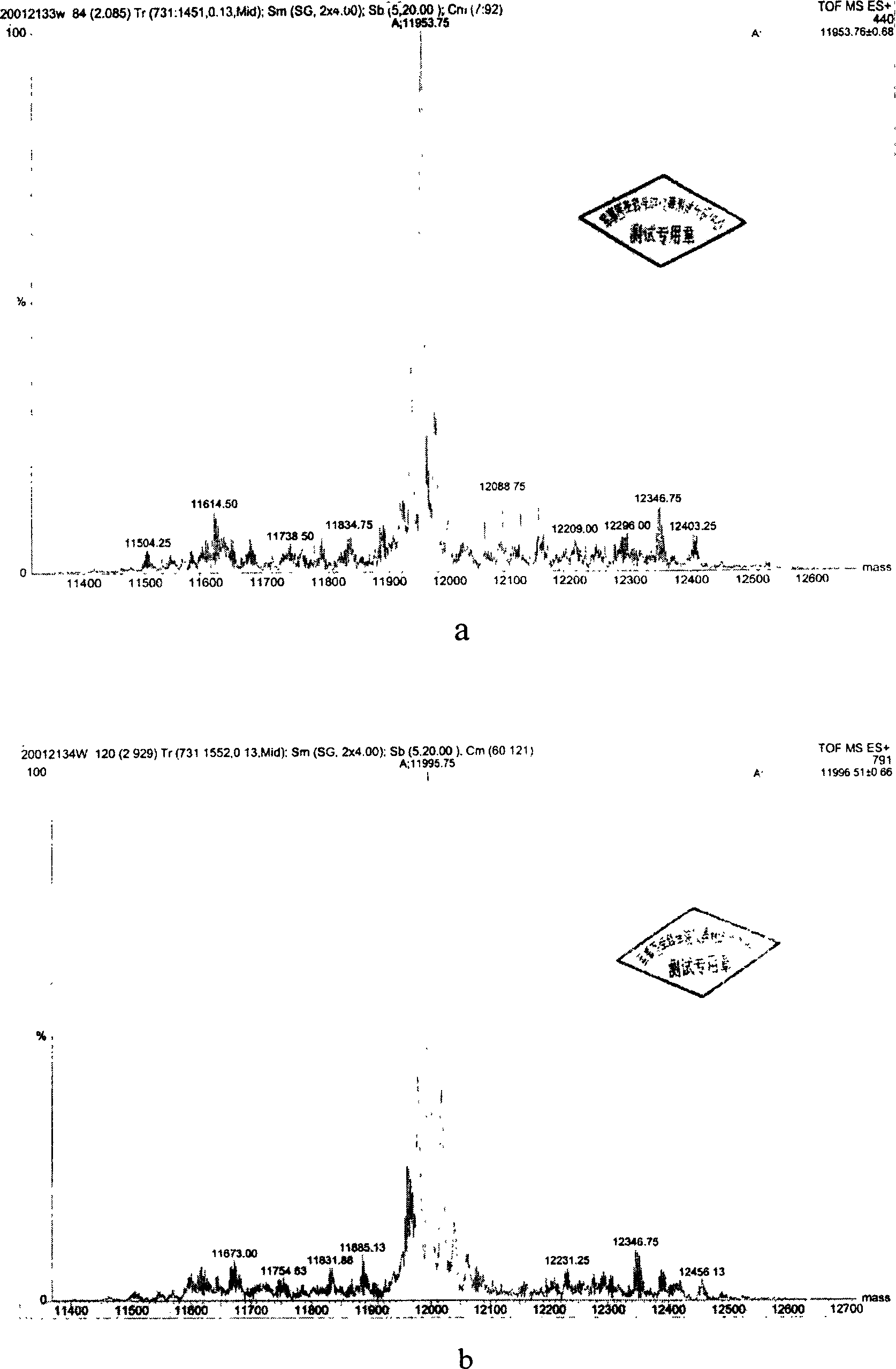
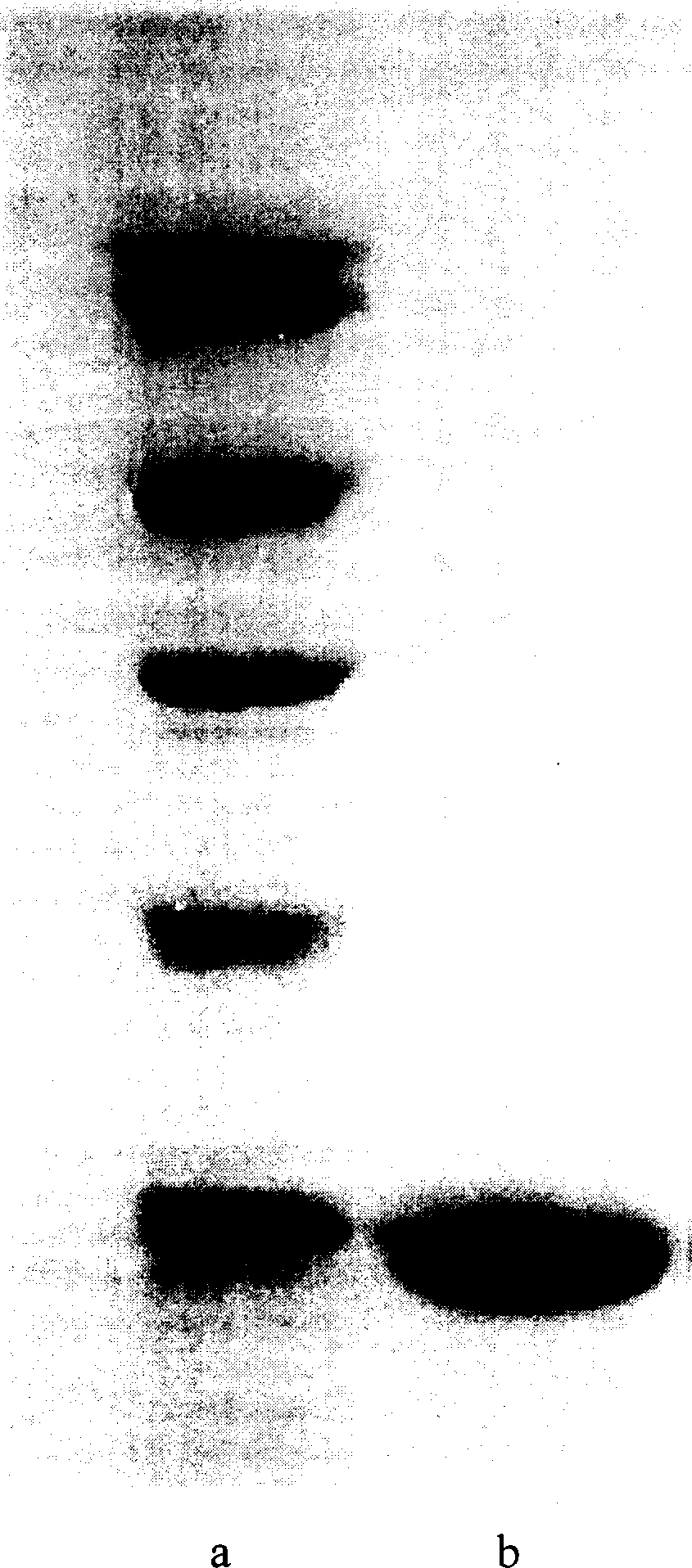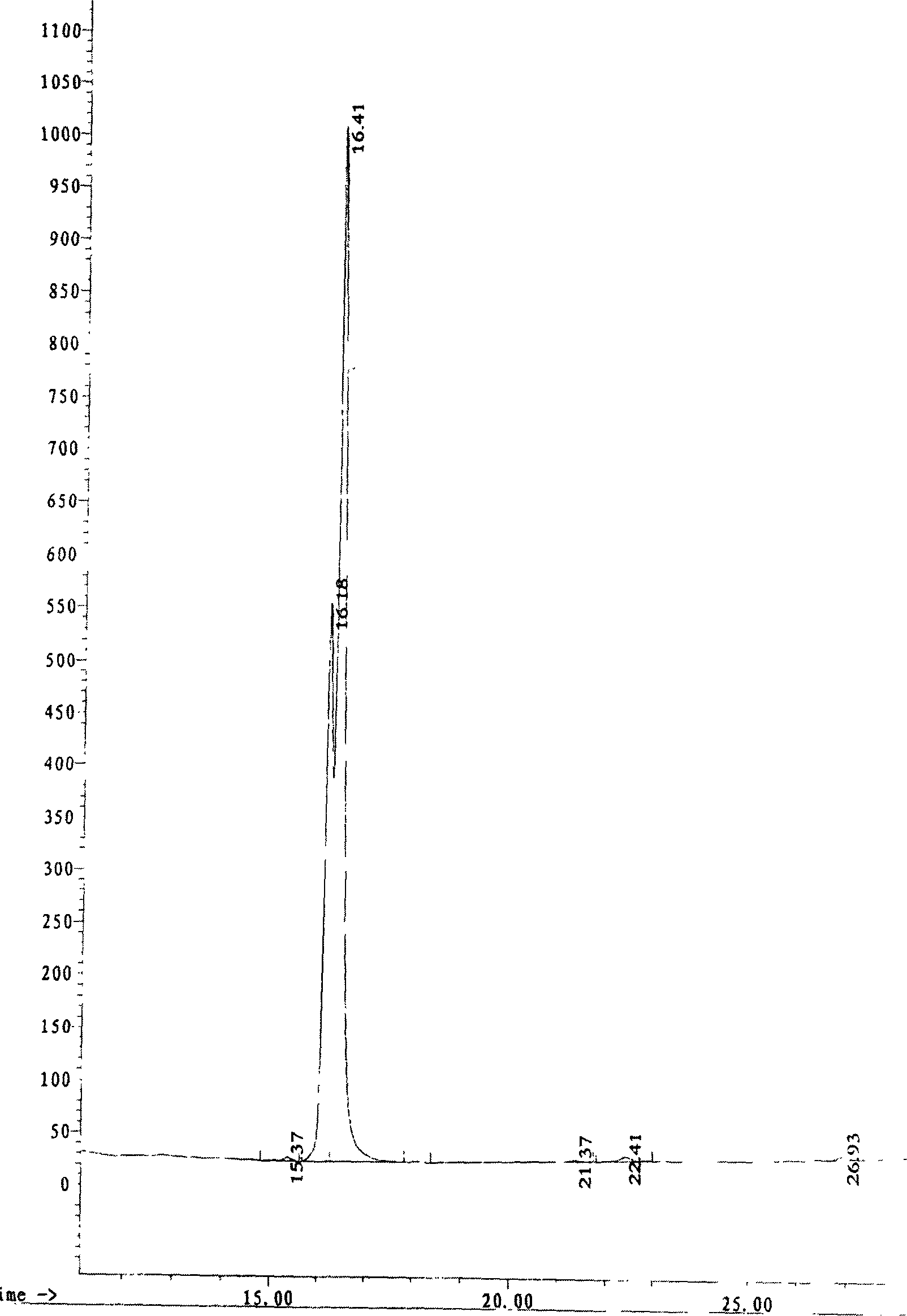Method for preparing N-end acetylation modified thymosin alpha with recombined E. coli
A technology for recombining Escherichia coli and Escherichia coli, which is applied in the field of preparing N-acetylated proteins, and can solve the problems of no thymosin α and no acetylated modified products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The prothymosin alpha (Ile) of embodiment one N-terminal acetylation 13 ) preparation
[0035] 1. Expression of prothymosin α (Ile 13 ) construction of recombinant Escherichia coli
[0036] The pfu enzyme, endonuclease, ligase, kit, DH5α, etc. in the experiment were purchased from Shanghai Sangon Bioengineering Technology Service Co., Ltd. and Beijing Biotech Biogene Technology Co., Ltd.
[0037] Take four-month-old aborted human fetal thymus, use total RNA preparation kit, prepare total RNA according to the method provided by the kit, use RT-PCR kit, reverse transcribe mRNA into cDNA according to the method provided by the kit, and use the cDNA as Template from which prothymosin alpha (Ile 13 ) cDNA. The primers Prot1 and Prot2 used were synthesized with an oligonucleotide synthesizer.
[0038] Prot1: cggaattcatgtctgatgcagctgtagataccagctccgaaatcaccatcaaggactta
[0039] Prot2: cgggatccctagtcatccacgtcggtcttctg
[0040] The PCR method is:
[0041] Add 1 μl cDN...
Embodiment 2
[0055] Example two N-terminal acetylated thymosin α1 (Ile 13 ) Preparation of analogues
[0056] The N-terminal acetylated prothymosin α (Ile 13 ), dissolved in 2mol / L of hydroxylamine hydrochloride, then adjusted the pH to 9.0 with 4.5mol / L LiOH, kept at 45°C in a water bath for 4h, and the product was separated by RP-HPLC (using HP1050 high pressure liquid chromatography, C18 column (4.6×250mm , Dalian Institute of Chemical Physics, Chinese Academy of Sciences), liquid A is pure water containing 0.1% TFA; liquid B is chromatographically pure acetonitrile (Tianjin Siyou Company) containing 0.1% TFA, fine gradient: 0→50min, A100%→10% , B 0% → 90%.), collect each elution peak, after freeze-drying respectively, use Q-TOF2 mass spectrometry to analyze, take the peptide segment that molecular weight is about 3136, namely N-terminal acetylated thymosin α1 (Ile 13 )analog. It has the same sequence as the N-terminal 28 amino acid residues of Sequence 1 in the sequence listing, and...
Embodiment 3
[0057] Thymosin α11 (Ile 13 ) Preparation of analogues
[0058] The N-terminal acetylated prothymosin α (Thr 13 ), dissolved in 2mol / L of hydroxylamine hydrochloride, then adjusted the pH to 9.0 with 4.5mol / L LiOH, kept at 45°C in a water bath for 4h, and the product was separated by RP-HPLC (using HP1050 high pressure liquid chromatography, C18 column (4.6×250mm , Dalian Institute of Chemical Physics, Chinese Academy of Sciences), liquid A is pure water containing 0.1% TFA; liquid B is chromatographically pure acetonitrile (Tianjin Siyou Company) containing 0.1% TFA, fine gradient: 0→50min, A100%→10% , B 0% → 90%.), collect each elution peak, after freeze-drying respectively, use Q-TOF2 mass spectrometry to analyze, take the peptide segment that molecular weight is about 3818, namely N-terminal acetylated thymosin α11 (Ile 13 )analog. It has the same sequence as the 35 amino acid residues at the N-terminal of Sequence 1 in the sequence listing, and the N-terminal has bee...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com