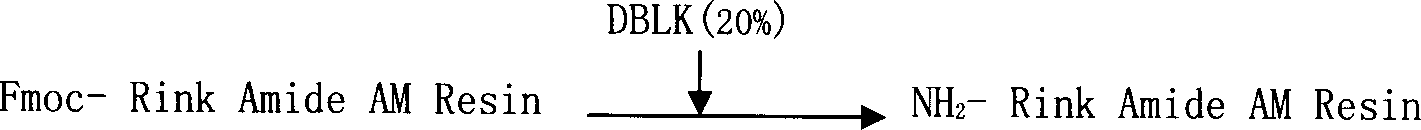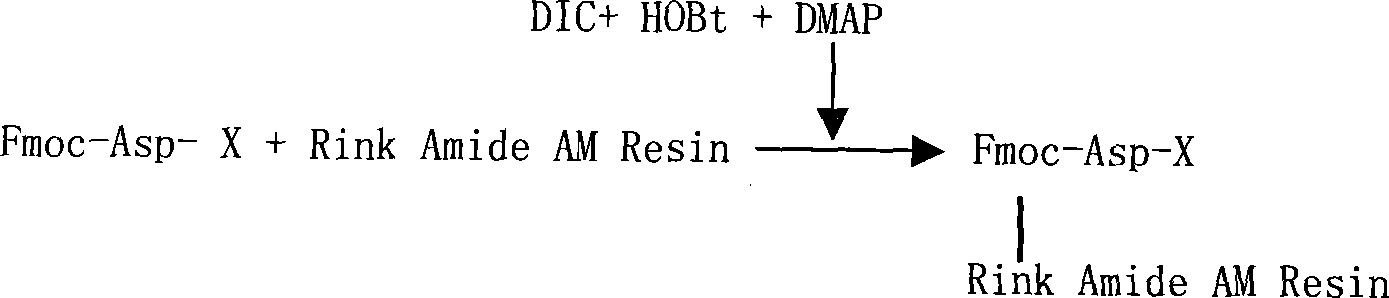Solid phase synthetic technique for thymosin alpha1
A technology of solid-phase synthesis and thymosin, which is applied in the field of thymosin α1, can solve the problems of violent reaction, high production cost, and low yield, and achieve the effects of mild reaction conditions, safety of production personnel, and reduction of production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
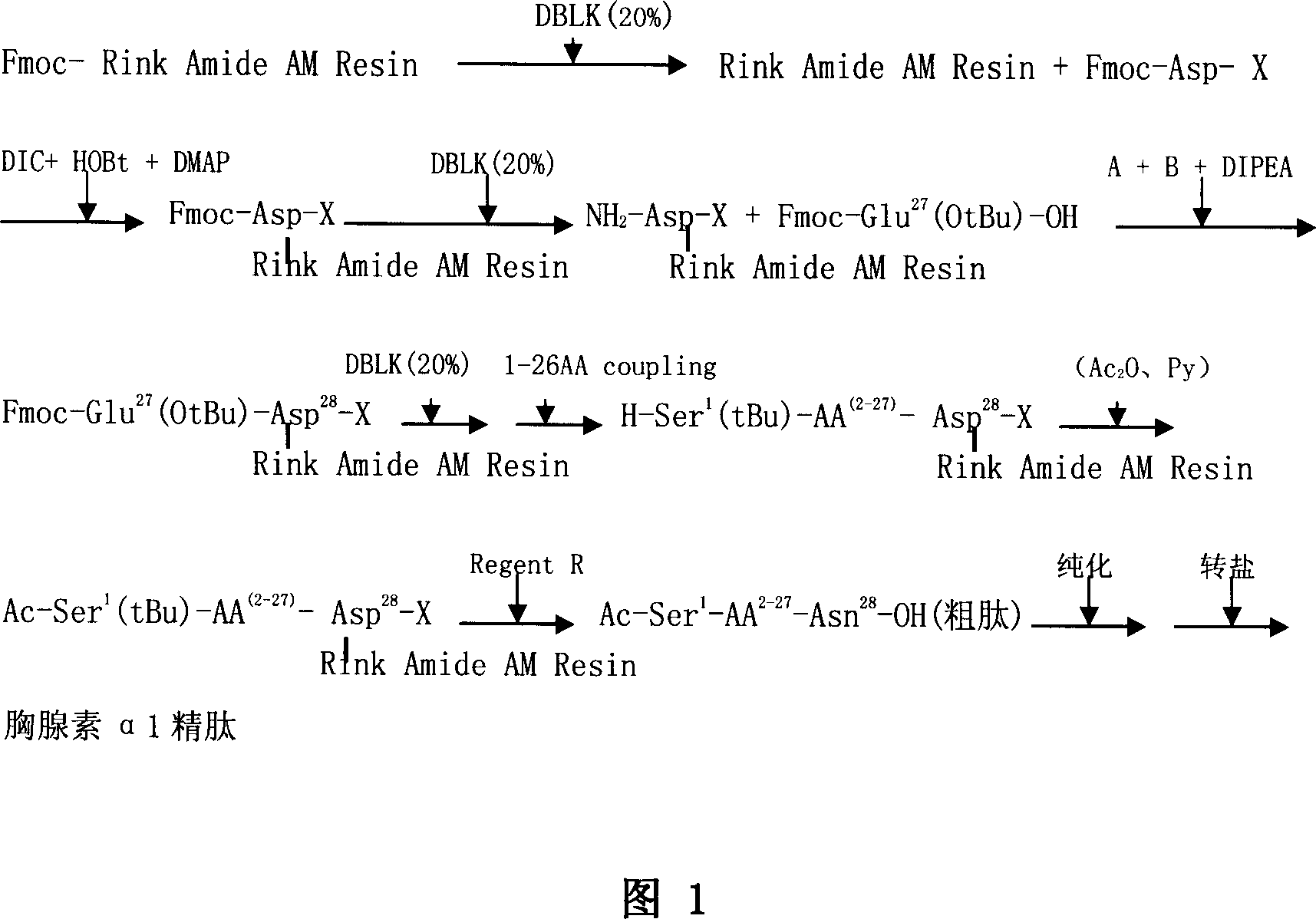
[0050] Embodiment 1: Synthesis of Fmoc-Asp (Rink Amide AM Resin)-OtBu
[0051] Add 2g of Fmoc-Rink Amide AM Resin, with a substitution degree of 1.1mmol / g, into a solid-phase reactor, add 20ml of DCM to swell the resin for 30min, and remove Fmoc protection twice with 20% DBLK5+10min to obtain NH 2 -Rink Amide AM Resin, washed 4 times in DMF and 2 times in DCM.
[0052] Dissolve 0.91g of Fmoc-Asp-OtBu, 0.4ml of DIC, and 0.327g of HOBt in 4ml of DMF, activate in ice water at low temperature for 10min, add to the above solid-phase reactor, and react at room temperature for 2h. After washing twice with DMF, 6.2 ml of acetic anhydride and 5.3 ml of pyridine were added to react for 4 hours to block unreacted free amino groups on the resin. After washing twice with DMF, it was shrunk with methanol for 10+10 min to obtain Fmoc-Asp(Rink Amide AM Resin)-OtBu, and the detected substitution degree was 0.453mmol / g.
Embodiment 2
[0053] Example 2: AA 1-27 -Synthesis of Asp(resin)-OtBu
[0054] Weigh 2.2g of Fmoc-Asp(Rink Amide AM Resin)-OtBu with a degree of substitution of 0.453mmol / g, add to the reactor and swell with DCM for 0.5 hours, then remove Fmoc with 20% DBLK (5+5)min, wash Then connect the 27th amino acid Fmoc-Glu(OtBu)-OH, dissolve 1.702g Fmoc-Glu(OtBu)-OH, 1.517gHBTU, 0.149gHOBt in 5mlDMF, add 1mlDIPEA after 10 ice baths; after low temperature activation for 10min, add the above solid In the phase reactor, react at room temperature for 1-2 hours, and the end point of the reaction is determined by the ninhydrin method. Repeat the above steps, and so on to complete the connection of amino acids from 26 to 1 to obtain AA 1-27 -Asp(resin)-OtBu. Raw material dosage: amino acid 1.0mmol, HBTU 1.517g, HOBt 0.149g, DIPE Alml.
Embodiment 3
[0055] Example 3: Ac-AA 1-27 -Synthesis of Asp(resin)-OtBu
[0056] Add 2.8ml of acetic anhydride and 2.4ml of pyridine in the reactor, and react at room temperature for 4 hours. The ninhydrin method resin is colorless and transparent, and the reaction is complete. The acetylation reagent is removed, washed twice with DMF, and shrunk with methanol (10+10 )min, Ac-AA was obtained after drying under reduced pressure for 6 hours 1-27 -Asp(resin)-OtBu 4.9 g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com