Escherichia coli overexpressing riml and its application in the preparation of n-acetylated thymosin α
A thymosin, the technology in the sequence table, applied in the biological field, can solve the problems of high product price, limited wide application, low yield, etc., and achieves the effects of convenient separation, obvious application prospects, and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1, the construction of the Escherichia coli overexpressing RimL expressing thymosin α
[0059] 1. Construction of Escherichia coli overexpressing N-acetyltransferase RimL
[0060] 1. Construction of pOKBV plasmid
[0061] Due to the co-expression of RimL and Thymosin α, considering the incompatibility of the plasmid, the replicon and ampicillin resistance gene of pBV220 (purchased from Shanghai Jingjing Molecular Biotechnology Co., Ltd.) were replaced with the plasmid pOK12 (Jeffrey Vieira and Joachim Messing.New pUC-derived cloning vectors with different selectable markers and DNAreplication origins.Gene, 1991,100:189-194, can be constructed from the information provided by this document, and the construction method of the carrier has been disclosed in many documents (such as J . Sambrook et al., "Molecular Cloning Experiment Guide" Second Edition, Science Press, 1995; ) etc., so it can be obtained by using the method of gene total synthesis.) p15A replico...
Embodiment 2
[0084] Embodiment 2, recombinant bacterium BL21 (DE3) (pOKBV-RimL / proTα (Thr 13 )) expresses N-acetylated prothymosin α (Thr 13 )
[0085] 1. Induce recombinant bacteria
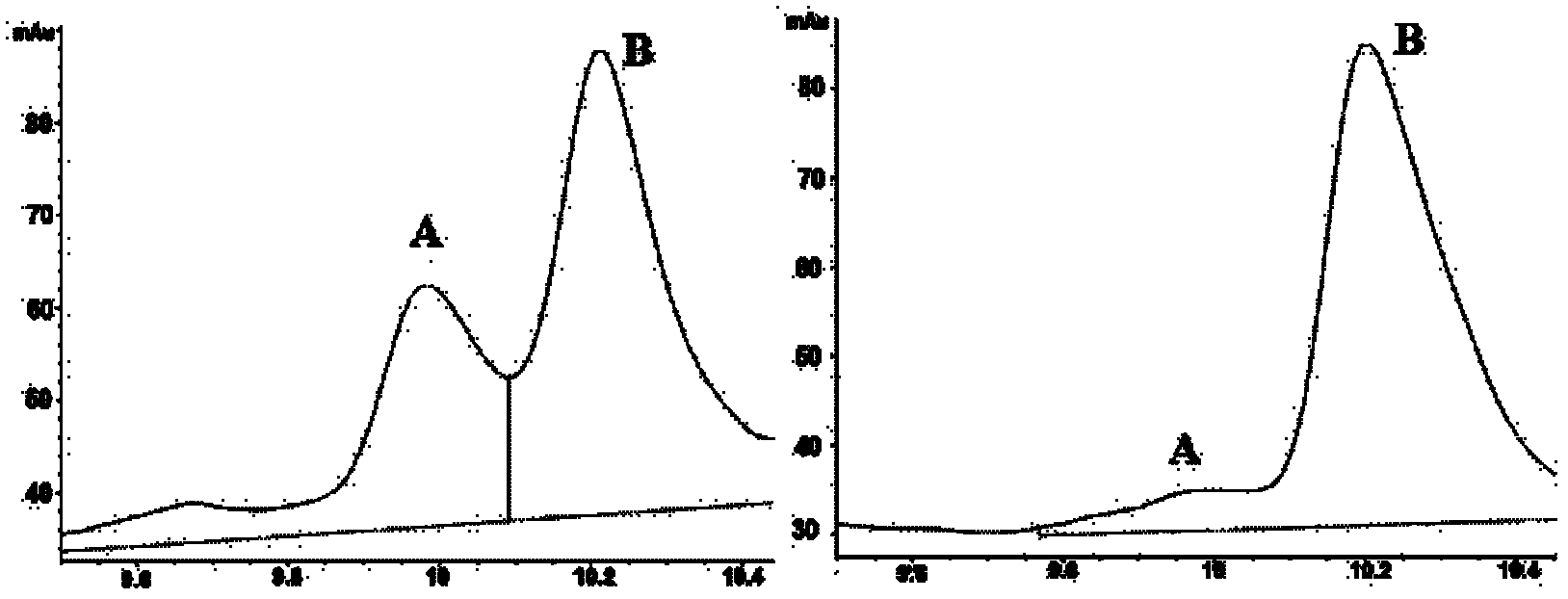
[0086] BL21(DE3)(pOKBV-RimL / proTα(Thr 13 )) and control bacteria BL21(DE3)(pOKBV / proTα(Thr 13 )) were inoculated into 5 mL No. I medium containing 50 μg / ml kanamycin and 100 μg / ml ampicillin (50 mM disodium hydrogen phosphate / sodium dihydrogen phosphate buffer, 20 g / L yeast extract, 10 g / L tryptone, 2g / L glucose), cultivated overnight at 30°C, and transferred the overnight cultured bacterial solution to 500ml shake flasks containing 150mL No. 600nm At about 0.6, raise the temperature to 42°C to induce expression, and centrifuge after 12 hours of induction (the centrifugation time is 20 minutes, the centrifugation temperature is 4°C, and the centrifugal force is 10,000g;) to harvest the bacteria respectively.
[0087] 2. Preparation of N-acetylated thymosin α progen (Thr 13 )
[0088]1) Tris saturated ...
Embodiment 3
[0099] Example 3, using the T7 promoter inserted into the genome to construct a gene containing and expressing N-acetylated thymosin α 13 ) recombinant bacteria
[0100] Using Red recombination technology, the T7 promoter with the lactose operon (derived from the PET22b plasmid, which was purchased from Novagen) was inserted into the open reading frame (ORF) of the N-acetyltransferase RimL gene of Escherichia coli BL21 (DE3). ), the T7 RNA polymerase gene controlled by the lactose promoter in the genome of the bacterium. When lactose or its analogs, such as IPTG, are added to the medium, the T7 RNA polymerase gene expression controlled by the lactose promoter, the synthetic T7 RNA polymerase binds to the T7 promoter artificially inserted into the upstream of the RimL gene open reading frame (ORF) At the same time, the lactose operon downstream of the T7 promoter is derepressed to control the high-efficiency transcription of the N-acetyltransferase RimL gene, thereby realizing...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com