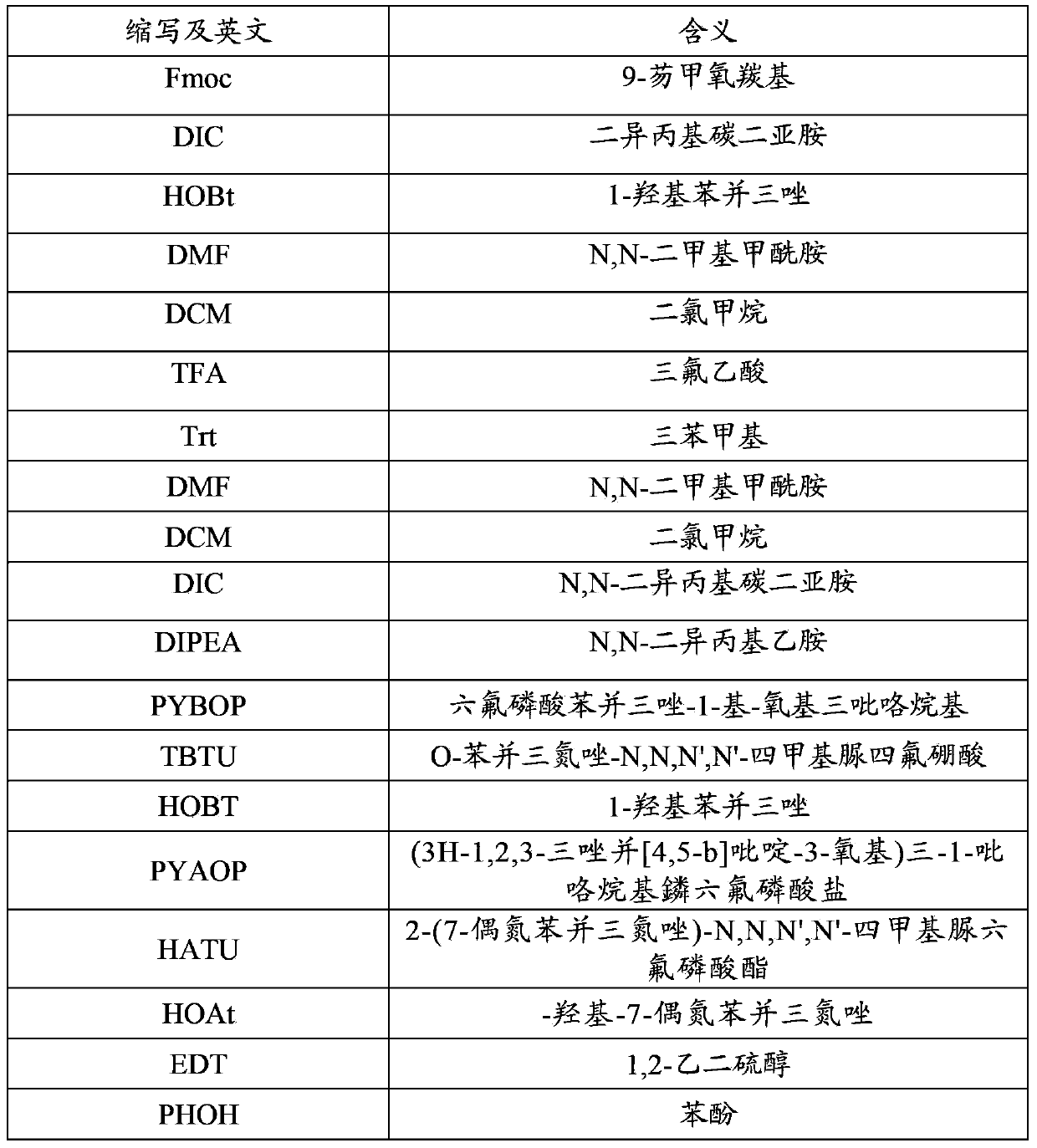
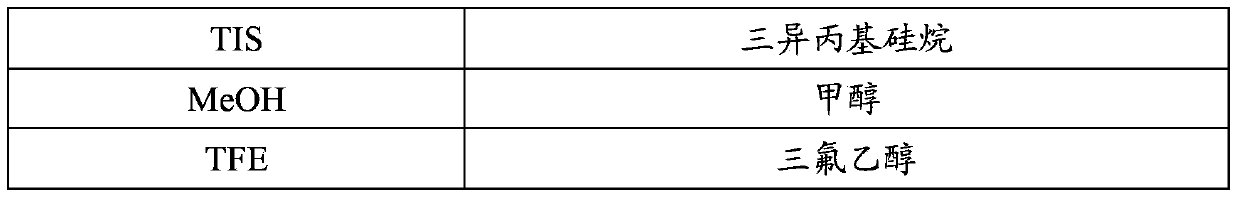
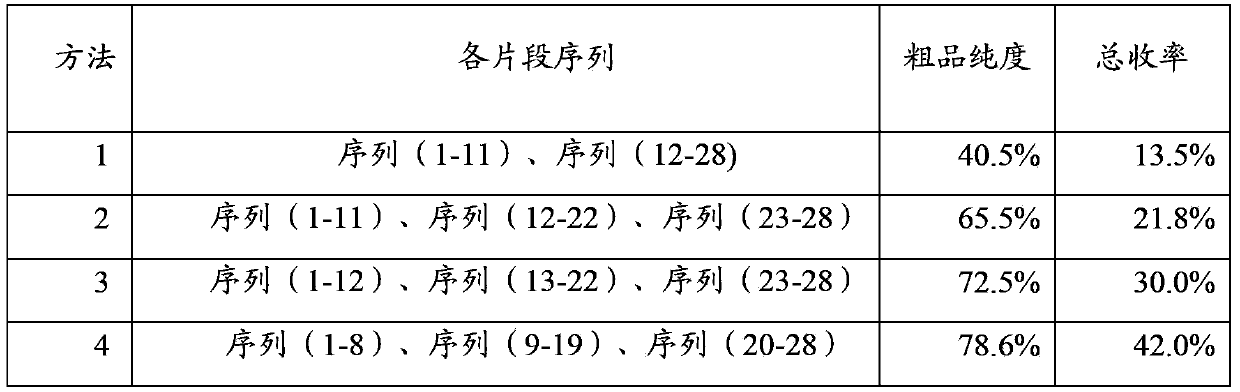
Method for synthesizing thymalfasin
A thymosin and solid-phase synthesis technology, which is applied in the preparation methods of peptides, thymosin, chemical instruments and methods, etc., can solve the problems that the yield and purity cannot reach a high level, there are many impurities, and the purification is difficult to carry out.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1: the preparation of polypeptide resin 1
[0071] 1. Preparation of Fmoc-β-Asp (OtBu)-Rink amide resin
[0072] Take Rink amide resin (20g, 16mmol) with a degree of substitution sub=0.8mmol / g, add it to the reaction column, swell it with DMF for more than 30 minutes, drain the DMF, and remove it with DBLK (a DMF solution containing 20% piperidine). Removal of the amino protecting group Fmoc of the resin was carried out for 5 minutes each time, twice in total. After the removal was completed, it was washed 6 times with DMF, drained and ready to be fed.
[0073] Weigh Fmoc-β-Asp (OtBu)-OH (6.6g, 16mmol), HOBt (2.6g, 19.2mmol), dissolve with DMF (40ml) and DCM (40ml), add DIC (2.4g, 19.2 mmol), activated for 5 minutes and then added to the reaction column. After 60 minutes, the reaction was completed. The resin was washed three times with DMF, and the blocking solution (pyridine (20ml) and acetic anhydride (22ml)) was added. Wash 6 times. Then add an appro...
Embodiment 2
[0076] Embodiment 2: Preparation of polypeptide fragment 1
[0077] Weigh 2-CTC resin (40g, 20mmol) with a substitution degree of 0.5mmol / g, add it to a solid-phase reaction column, wash it twice with DMF, and swell the resin with DMF for 30 minutes, then take Fmoc-Lys(Boc)- OH (28.1g, 60mmol) was dissolved in a mixed solution of DCM (30ml) and DMF (30ml) with a volume ratio of 1:1, and DIPEA (15.5g, 120mmol) was added under ice-cooling conditions, and the solution was added to the solid phase after activation for 5 minutes In the reaction column, react at room temperature for 2 hours. After the reaction, block with blocking solution (DIPEA: methanol: DCM = 1:2:17v:v) three times, each time for 3 minutes. The volume of closed liquid is calculated as 4.0ml / gram of resin. Then add an appropriate amount of methanol to wash 3 times, each time for 10 minutes, and wash 6 times with DMF. Fmoc protection was removed with DBLK, followed by 6 washes with DMF.
[0078] Dissolve Fmoc-G...
Embodiment 3
[0080] Embodiment 3: Preparation of polypeptide fragment 2
[0081] Weigh 2-CTC resin (40g, 20mmol) with a substitution degree of 0.5mmol / g, add it to a solid-phase reaction column, wash it twice with DMF, and swell the resin with DMF for 30 minutes, then take Fmoc-Ser(tBu)- OH (23.0g, 60mmol) was dissolved in a mixed solution of DCM (30ml) and DMF (30ml) with a volume ratio of 1:1, and DIPEA (15.5g, 120mmol) was added under ice-cooling conditions, and the solution was added to the solid phase after activation for 5 minutes In the reaction column, react at room temperature for 2 hours. After the reaction, use blocking solution (DIPEA: methanol: DCM = 1:2:17v:v) to block three times, each time for 3 minutes. The volume of closed liquid is calculated as 4.0ml / gram of resin. Then add an appropriate amount of methanol to wash 3 times, each time for 10 minutes, and wash 6 times with DMF. Fmoc protection was removed with DBLK, followed by 6 washes with DMF.
[0082] Dissolve Fmoc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com