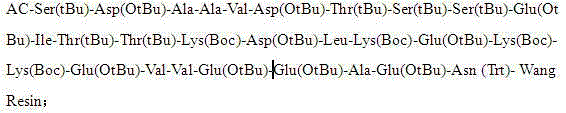Method for preparing thymalfasin through dipeptide fragment liquid-solid bonding
A novel thymus method, liquid-solid combination technology, applied in the preparation method of peptides, thymosin, chemical instruments and methods, etc., can solve the problems of coupling difficulties, reduce the cost of purification, avoid the generation of missing peptides, and benefit industrial scale The effect of mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Preparation of Fmoc-Lys(Boc)-OSu
[0052] Accurately weigh 234.2 g (0.5 mol) of Fmoc-Lys(Boc)-OH and 57.5 g (0.5 mol) of HOSu, dissolve them in 1000 ml THF, and stir in an ice-water bath. Accurately weigh 103.2 g (0.5 mol) of DCC, dissolve it in 600 ml tetrahydrofuran, slowly add it dropwise to the above solution, stir in an ice bath for 1 h, then continue to react at 25°C for 2 h. After the reaction is completed, filter with suction, concentrate the filtrate to 500-600 ml by rotary evaporation, add 2000 ml of petroleum ether, a large amount of white solid is precipitated, and filter with suction. The filter cake was dissolved in 500 ml of ethyl acetate, 1500 ml of petroleum ether was added, the solution was clarified, and it was placed in a -20 degree refrigerator. After 2 h, a large amount of white solids were precipitated, filtered with suction, and the filter cake was dried and weighed to obtain Fmoc-Lys ( Boc)-OSu 263.1g, yield 93%.
Embodiment 2
[0053] Example 2: Preparation of Fmoc-Lys(Boc)-Lys(Boc)-OH
[0054] Accurately weigh 110.8 g (0.45 mol) of H-Lys(Boc)-OH and 57.2 g (0.54 mol) of sodium carbonate and dissolve it in 1000 mL of water, slowly add Fmoc-Lys(Boc)-OSu under ice bath (2-8°C) (254.5 g, 0.45 mol) in 800 ml of tetrahydrofuran solution, stirred for reaction, and TLC monitored the end point of the reaction. After the reaction is complete, remove tetrahydrofuran by rotary evaporation under reduced pressure, add 10% citric acid aqueous solution to the remaining aqueous solution in an ice-water bath to adjust the pH value of the solution to 2-3, extract three times with 2000ml ethyl acetate, combine the organic phases, and concentrate to 1000ml, 600ml by rotary evaporation Wash with saturated brine three times, dry over anhydrous sodium sulfate, add 1000 ml of petroleum ether for crystallization, and obtain 280.1 g of Fmoc-Lys(Boc)-Lys(Boc)-OH, with a yield of 89.1%.
Embodiment 3
[0055] Example 3: Preparation of Fmoc-Thr(tBu)-OSu
[0056] Accurately weigh 198.74 g (0.5 mol) of Fmoc-Thr(tBu)-OH and 63.25 g (0.55 mol) of HOSu, dissolve them in 1000 ml THF, and stir in an ice-water bath. Accurately weigh 113.5 g (0.55 mol) of DCC, dissolve it in 600 ml tetrahydrofuran, slowly add it dropwise to the above solution, stir in an ice bath for 1 h, and then continue to react at 25°C for 2 h. After the reaction is completed, filter with suction, concentrate the filtrate to 500-600 ml by rotary evaporation, add 2000 ml of petroleum ether, a large amount of white solid is precipitated, and filter with suction. The filter cake was dissolved in 500 ml of ethyl acetate, 1500 ml of petroleum ether was added, the solution was clarified, and it was placed in a refrigerator at -20 degrees. After 2 h, a large amount of white solids were precipitated, filtered by suction, and the filter cake was dried and weighed to obtain Fmoc-Thr( tBu)-OSu 234.85g, yield 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com