Hybrid peptide with functions of regulating immunity, resisting oxidation and inflammation and detoxifying body, and preparation method and application thereof
A hybrid peptide and antioxidant technology, applied in the fields of genetic engineering and biological preparations, can solve the problems of increasing the complexity and antagonism of clinical drug selection, failing to improve the immune function of the body, and reducing immunity and production performance, and achieving weight reduction. As well as intestinal damage, good immune and anti-inflammatory two-way regulation, and the effect of relieving tissue damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 Preparation of immune anti-inflammatory hybrid peptide LTP
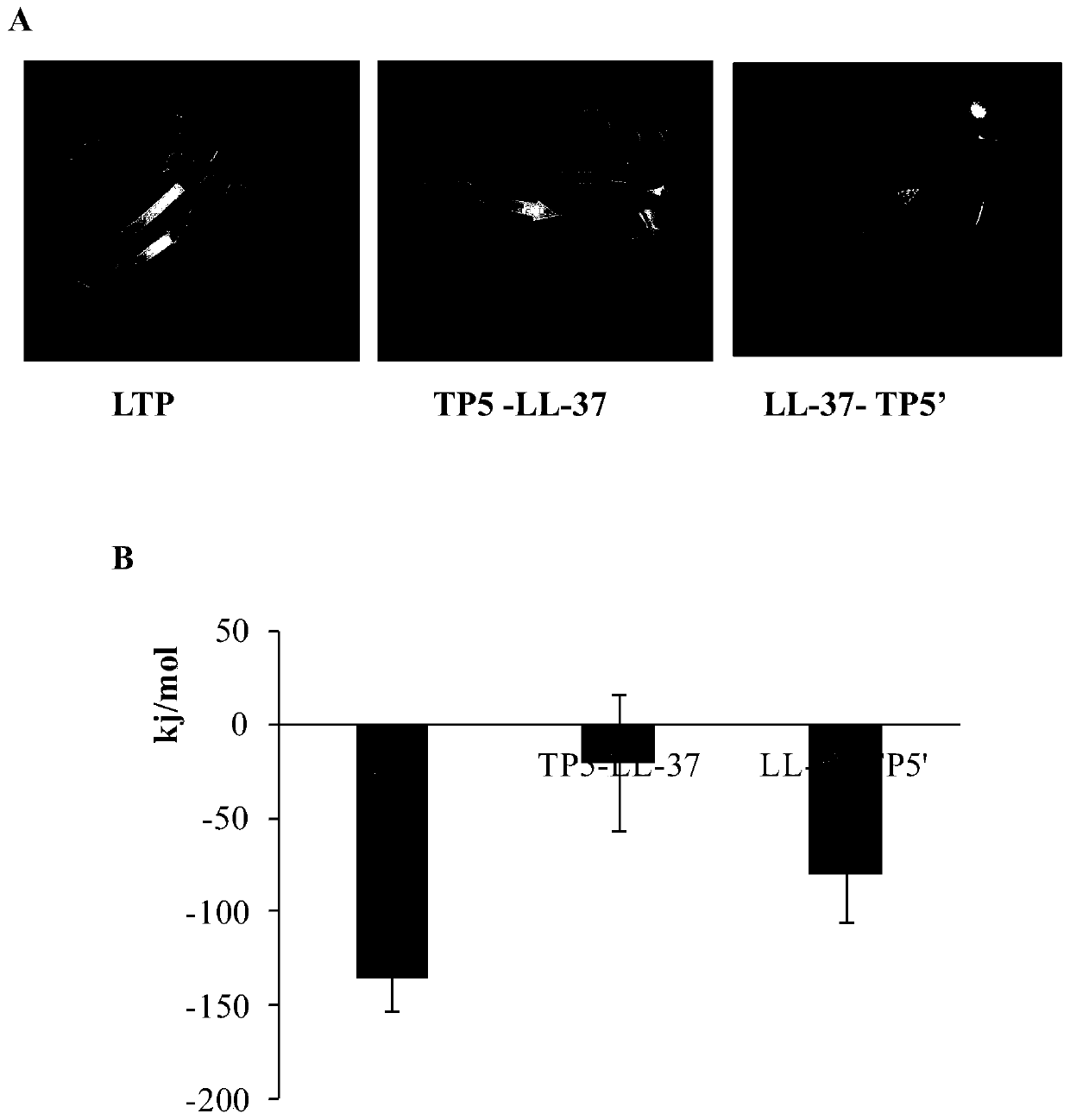
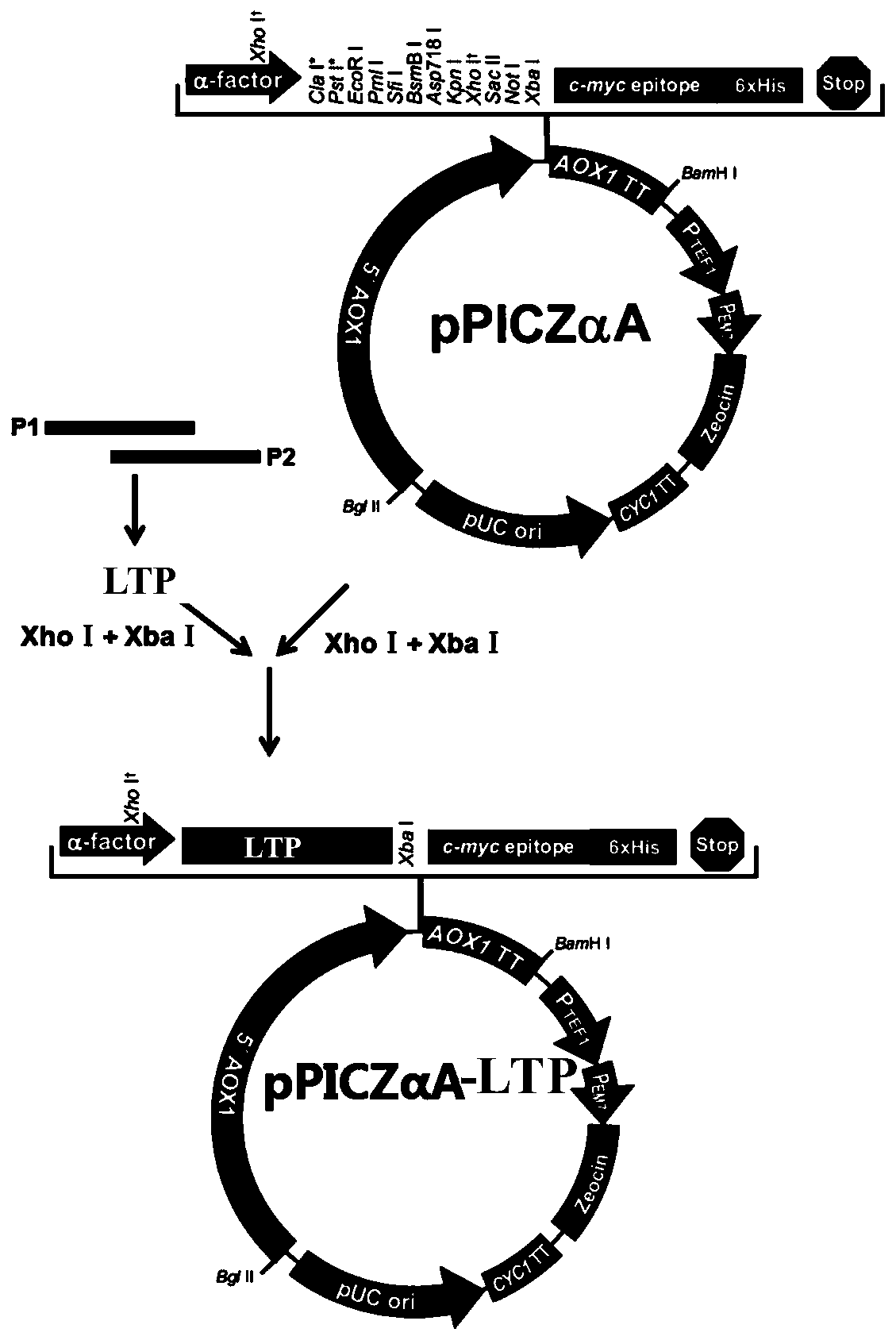
[0056] 1. Obtaining the hybrid peptide sequence
[0057] Through the research on the sequence, structure and the relationship between sequence structure and function of polypeptide LL-37 and thymopentin TP5, the hybridization of polypeptide LL-37 and thymopentin TP5 was carried out by protein molecular design technology, and the candidate hybrid peptide LTP was obtained , TP5-LL-37, and LL-37-TP5', the amino acid sequences of which are shown in SEQ ID NO.1, SEQ ID NO.5, and SEQ ID NO.6, respectively. Myeloid differentiation protein-2 (MD-2) binds to TLR4 and endows TLR4 with responsiveness to various ligands (including LPS); secondly, MD-2 can promote the expression of TLR4 and TLR2, and interact with TLR4 in cells distribution are closely related. Therefore, MD-2 is not only an auxiliary molecule of TLR4, but also a regulatory molecule in innate immunity, which may have a wider range of biological...
Embodiment 2
[0071] Example 2 The neutralization effect of hybrid peptide LTP on LPS
[0072] Use pyrogen-free endotoxin test water to dissolve and dilute the hybrid peptide LTP and its parent peptides LL-37 and TP5 into solutions of different concentrations (0-64 μg / mL), and take 100 μL of the above concentrations of LTP polypeptide solution and LPS respectively. (1EU / mL) mixed. After incubating at 37°C for 30 min, the neutralization rate of polypeptide LTP, LL-37, and TP5 to LPS was detected using a chromogenic mechanism Limulus kit, and polymyxin B (PMB) was used as a control. The result is as Figure 5 As shown, the polypeptide LTP has a higher LPS neutralizing activity, and its LPS neutralizing activity is equivalent to that of polymyxin B. When the concentration is 8 μg / mL, the neutralization rate of the hybrid peptide LTP to LPS is close to 100%, and Its neutralizing activity was significantly higher than that of its parent peptides LL-37 and TP5.
Embodiment 3
[0073] Example 3 Effect of Hybrid Peptide LTP on Survival Rate of Mouse Macrophage Cells
[0074] The macrophage RAW264.7 in the logarithmic growth phase was inoculated in a 96-well plate, and the initial cell culture density was 1×10 4 cells / mL, 100 μL per well, at 37°C, 5% CO 2 After culturing overnight under certain conditions, a series of LTP, LL-37, and TP5 (0-100 μg / mL) solutions were added in a series of concentration gradients. After culturing for 24 hours, the effect of polypeptide LTP on the survival rate of mouse macrophages was detected by the CCK8 method. . The result is as Figure 6 As shown, the cytotoxicity of the hybrid peptide LTP is significantly lower than that of its parent peptide LL-37, and the survival rate of macrophages is greater than 80% in the concentration range of 0-100 μg / mL, indicating that the cytotoxicity of LTP is low and has Higher security.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com