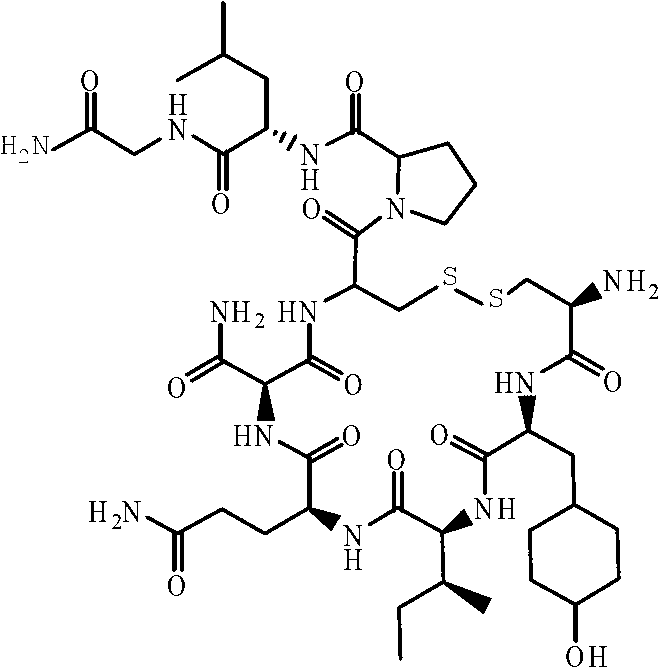
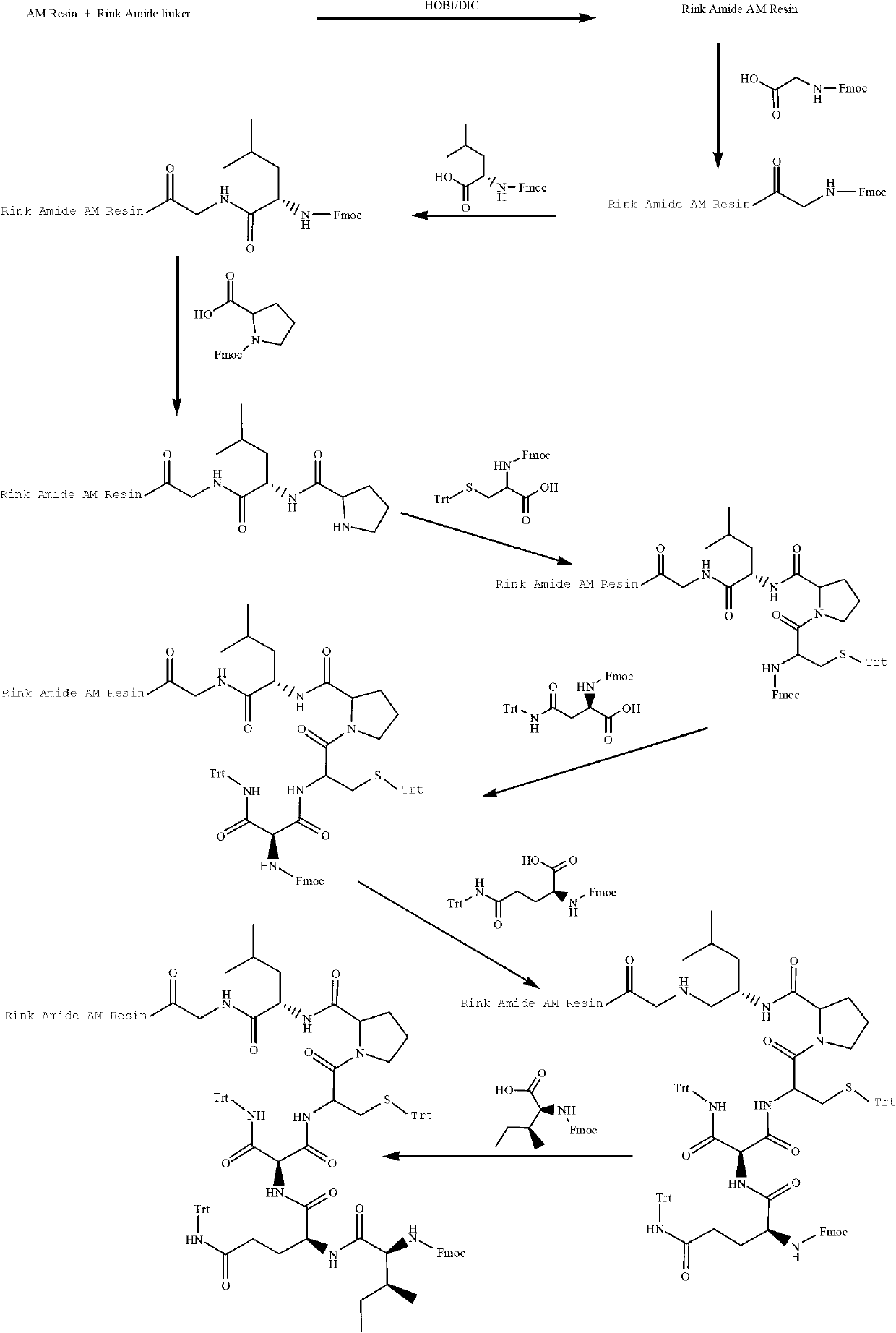
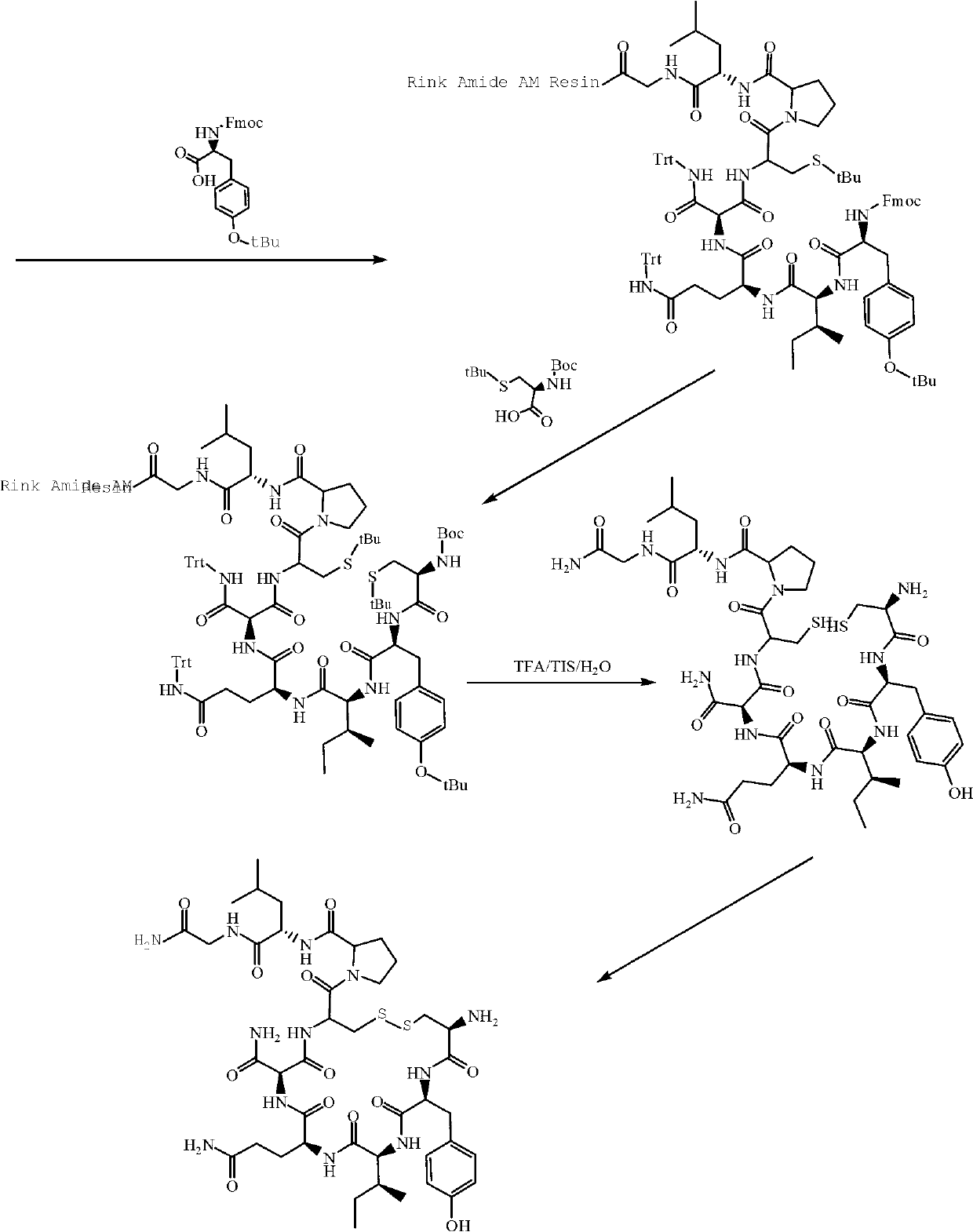
Solid-phase synthetic method of oxytocin
A solid-phase synthesis method and solid-phase synthesis technology, which are applied in the preparation methods of peptides, chemical instruments and methods, oxytocin/vasopressin, etc., can solve the problem of reduced yield and purity of cyclic peptides, long reaction time, The post-processing is cumbersome and other problems, to achieve the effect of low cost, little environmental pollution and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A solid-phase synthesis method of oxytocin, comprising the following steps:
[0026] Step (1) Preparation of Rink Amide AM Resin
[0027] Weigh 10g of AM Resin (100 mesh, 0.49mmol / g), soak in 50mL DCM for 30min to fully swell the resin, and then wash twice with 50mL DMF (not less than 1min each time). Weigh Rink Amide Linker: 7.93g, HOBt: 1.98g, DIC: 2.24mL, add to 10mL of anhydrous DMF, activate at 0~5℃ for 15min. Add solid-phase synthesis tubes and react at 30°C for 2.0~4.0h. The end point of the reaction is determined by the ninhydrin method. After the reaction is complete, wash with 50mL of anhydrous DMF twice, each time not less than 1.0min. After testing the degree of substitution, The degree of substitution was 0.48mmol / g Rink Amide AM Resin.
[0028] Step (2) Preparation of Boc-Cys(Trt)-Tyr(tBu)-Ile-Gln(Trt)-Asn(Trt)-Cys(Trt)-Por-Leu-Gly-Rink Amide AM Resin
[0029] De-Fmoc protecting group
[0030] Use 70 mL of 25% piperidine / DMF to remove the Fmoc protecti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com