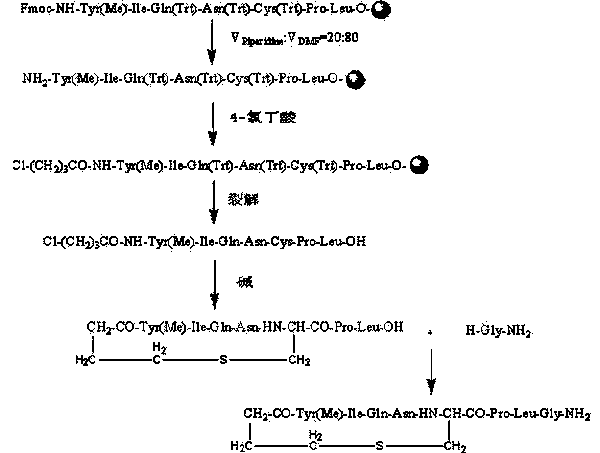
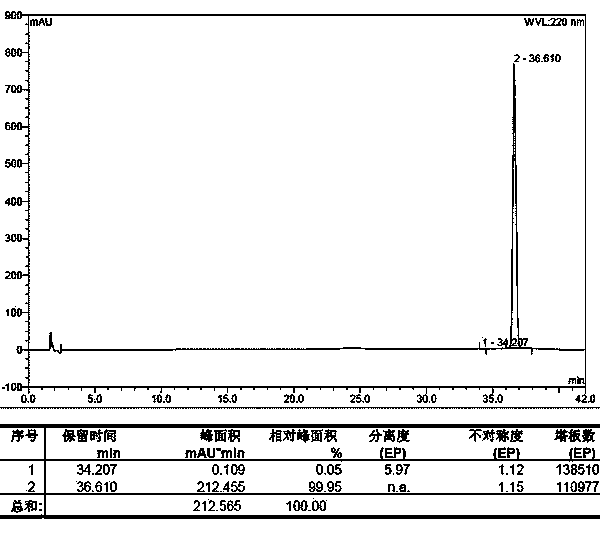
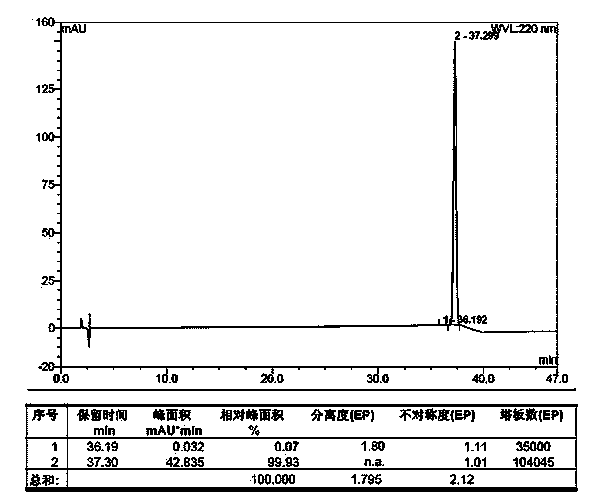
Carbetocin synthesis method
A carbetocin, solid-phase synthesis technology, which is applied in the preparation methods of peptides, chemical instruments and methods, oxytocin/vasopressin, etc. Not applicable to industrial production and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] (1) Amino acid resin synthesis: Weigh 1.25g (1.0mmol) Wang Resin (substitution degree of 0.80mmol / g) into the reaction column, add 15ml DCM, stir and swell at 15-35°C for 30min, and remove the liquid by suction filtration . 1.06 g (3.0 mmol) of Fmoc-Leu-OH and 0.41 g (3.0 mmol) of HOBt were weighed into the dissolution tank. Measure 6ml of DMF solvent into the tank, stir to dissolve, after complete dissolution, cool down to about 0-15°C in an ice bath, add 464μl (3.0mmol) of DIC, stir evenly, control the temperature at 10-25°C for 5min, add 0.04g (0.3 mmol) DMAP, then added to the reaction column, reacted at 25-35 ° C for 5 h, sampling to detect the substitution degree of 0.63 mmol / g, and the reaction solution was removed. Add 10 ml of DMF to the reaction column, stir and wash for 3 minutes, and repeat the washing 3 times. Measure 525 μl (5.5 mmol) of acetic anhydride, 445 μl (5.5 mmol) of pyridine, and 10 ml of DMF solution, mix them evenly, add them into the reactio...
Embodiment 2
[0127] Example 2: 100 mmol
[0128] (1) Amino acid resin synthesis: Weigh 125g (100.0mmol) of Wang Resin (with a substitution degree of 0.80mmol / g) and add it to the reaction column, add 1.4L DCM, stir and swell at 15-35°C for 30min, and remove the liquid by suction filtration . Weigh 106 g (300 mmol) of Fmoc-Leu-OH and 41 g (300 mmol) of HOBt into a dissolution tank. Measure 500ml of DMF solvent into the tank, stir to dissolve, after complete dissolution, cool down to about 0-15°C in an ice bath, add 46.4mL (300mmol) of DIC, stir evenly, control the temperature at 10-25°C for 5min, add 4.0g (30 mmol) DMAP, then added to the reaction column, reacted at 25-35 ° C for 5 h, sampling to detect the substitution degree of 0.63 mmol / g, and removing the reaction solution. Measure 900ml of DMF and add it to the reaction column, stir and wash for 3 minutes, and repeat the washing 3 times. Measure 52.5 mL (550 mmol) of acetic anhydride, 44.5 mL (550 mmol) of pyridine, and 900 mL of DM...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com