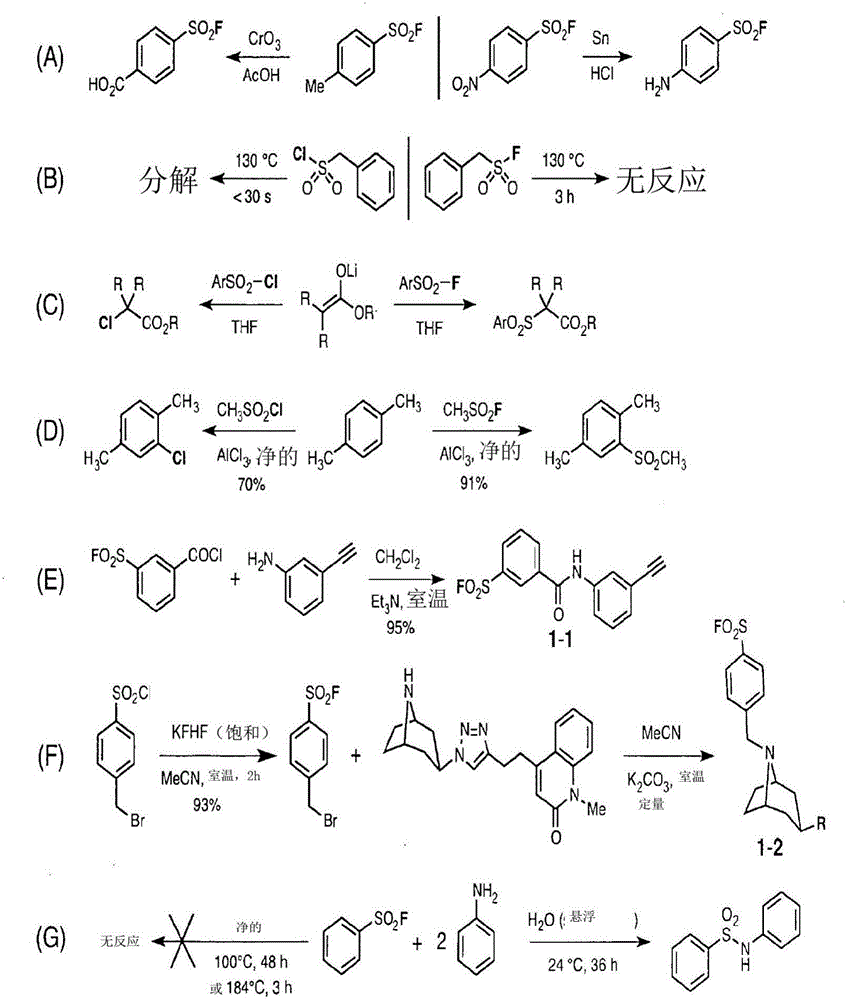
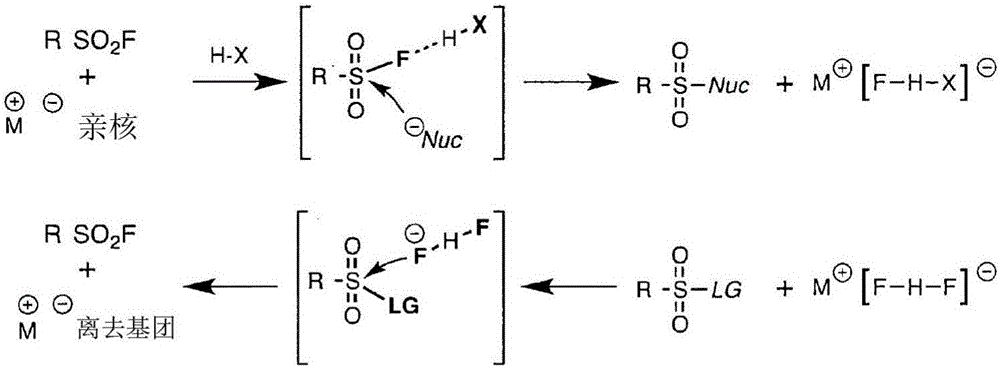
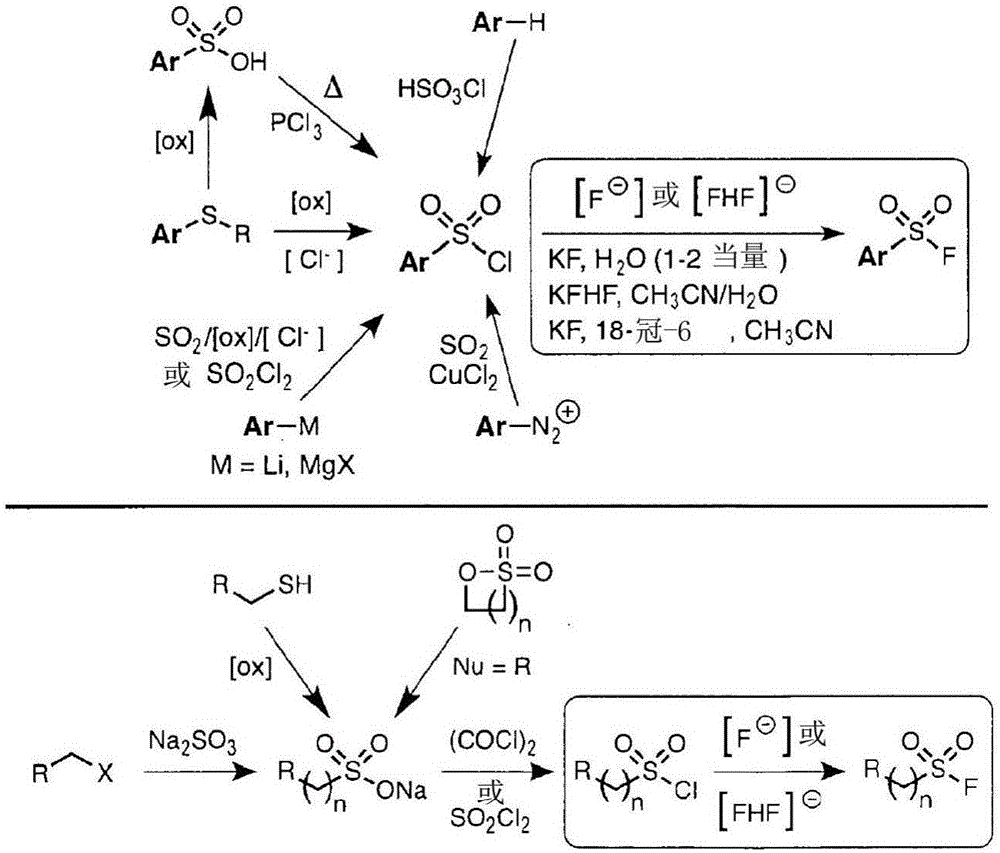
Sulfur(vi) fluoride compounds and methods for the preparation thereof
A technology of compounds and substances, applied in the field of preparing said compounds and compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0248] According to one embodiment, the pharmaceutical combination of the present invention suitable for vaginal administration is provided in the form of a diaphragm, tampon, cream, gel, ointment, foam or sponge, which comprises the compound of formula (I) of the present invention. Compounds and carriers known in the art. Alternatively, compositions suitable for vaginal administration may be delivered in liquid or solid dosage form. Additives, excipients, etc. are generally included in compositions for vaginal administration in concentration ranges suitable for their intended use or function in the composition, as is known in the art of pharmaceutical formulation. The compounds of formula (I) are contained in the compositions in a therapeutically useful and effective concentration range as determined by routine methods known in the medical and pharmaceutical arts. For example, a typical composition may contain one or more compounds of formula (I) in a concentration ranging f...
example 1
[0295] Example 1 (A) for short.
[0296] BEMP=2-tert-butylimino-2-diethylamino-1,3-dimethylperhydro-1,3,2-diazaphosphine, DBN=1,5-diazabicyclo[4.3 .0]non-5-ene, DBU=1,8-diazabicyclo[5.4.0]undec-7-ene, LHMDS=bis(trimethylsilyl)amide lithium, TCEP=tri( 2-carboxyethyl)phosphine hydrochloride, TMS = trimethylsilyl, TBS = tert-butyldimethylsilyl chloride.
[0297] Example 1 (B) General method.
[0298] Recorded on Bruker DRX-500, Bruker DRX-600, Bruker AMX-400 instruments 1 H and 13 C NMR spectra, and chemical shifts (δ) expressed in parts per million, as residual CHCl 3 , acetone, acetonitrile or DMSO as internal standard. Proton Magnetic Resonance ( 1 (HNMR) spectra were recorded at 600, 500 or 400 MHz. carbon magnetic resonance ( 13 C NMR) spectra were recorded at 150, 125 or 101 MHz. Fluorine magnetic resonance ( 19 F NMR) spectra were recorded at 376 MHz. NMR acquisitions were performed at 295K unless otherwise stated. The abbreviations are: s, singlet; d, doublet;...
example 2
[0456] Example 2 Modified Antibiotics
[0457] ArOSO 2 F is a non-polar functional group on the aromatic ring. It is an electrophile capable of coexisting with nucleophiles and capable of sustaining biological systems. ArOSO 2 F is very stable and can selectively react with different protein targets. Its non-polar functionality means that the introduction of functional groups on the parent has minimal or no effect on the affinity of the parent molecule.
[0458] Any known small molecule drug with one or more aromatic substitutions can be easily converted to ArOSO 2 F. Many antibiotics include functional groups such as aryl-OH, amino groups, etc., which can be derivatized to introduce SO 2 F group (such as OSO 2 F. NCH 2 CH 2 SO 2 F or NSO 2F) into the antibiotic structure. In this study, five fluorosulfonyl antibiotic derivatives (cephalosporin derivative 10-2, ciprofloxacin derivative 10-7 and three vancomycin derivatives - vancomycin-SF, vancomycin Mymycin-SF-1 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com