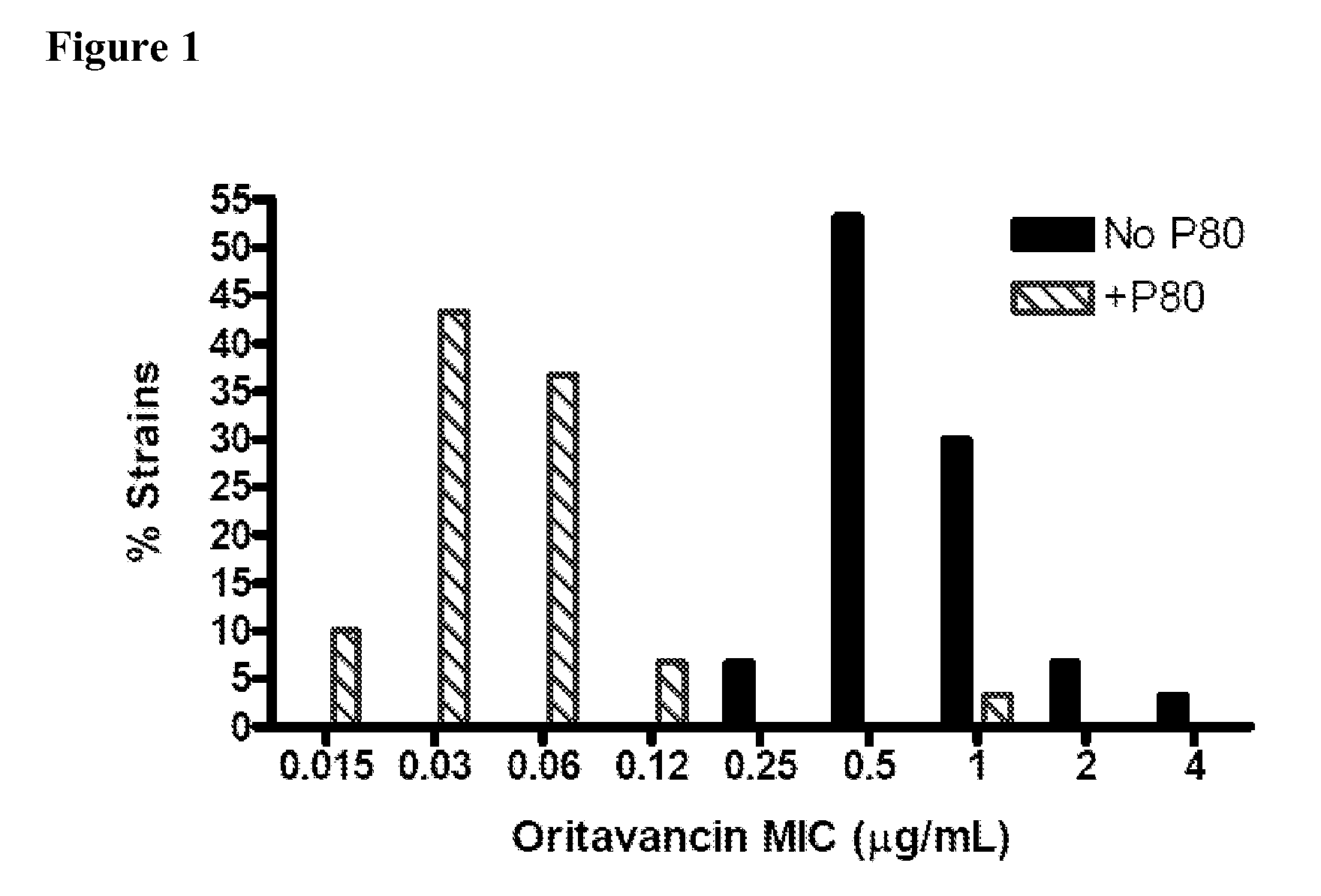
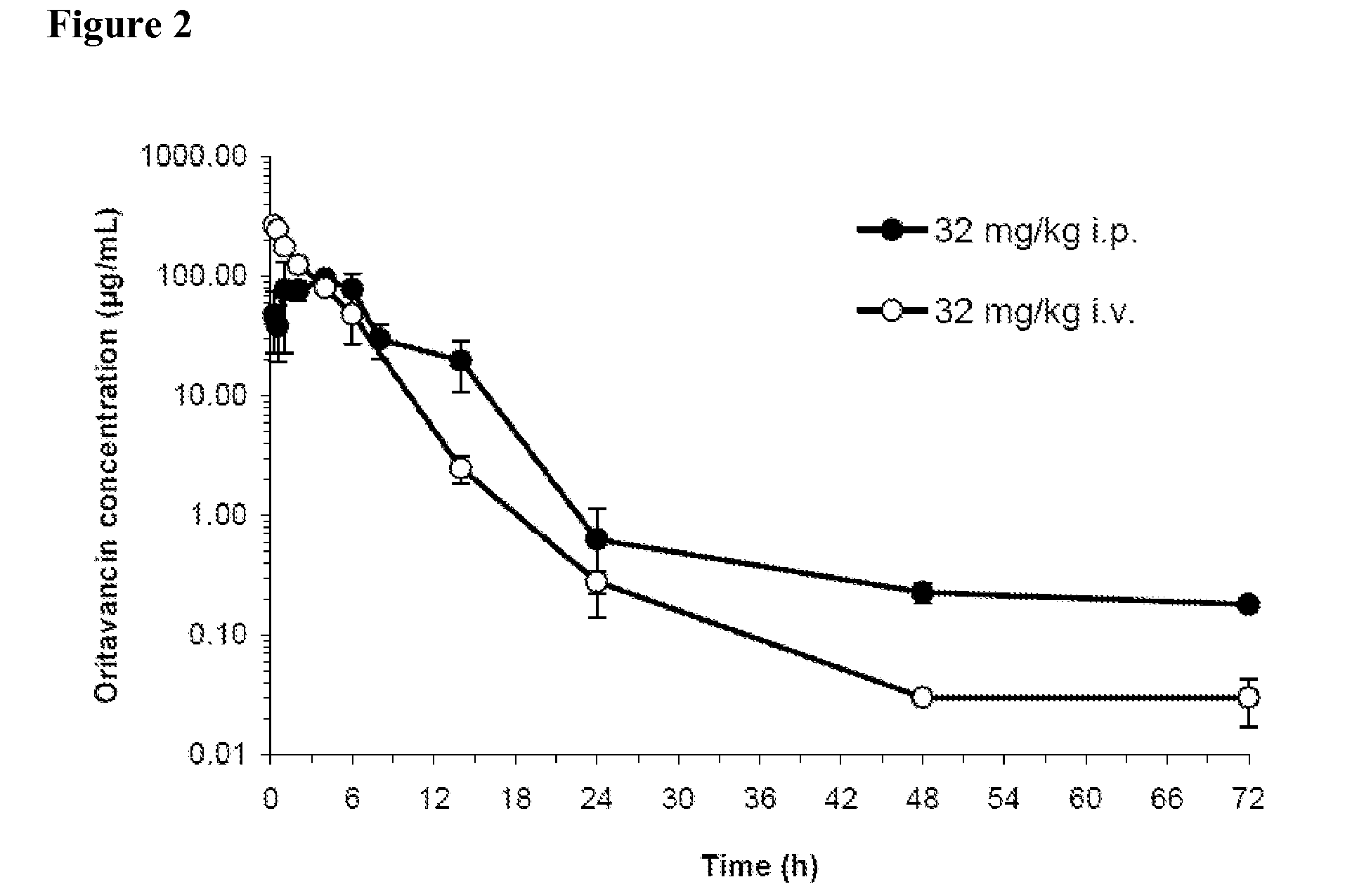
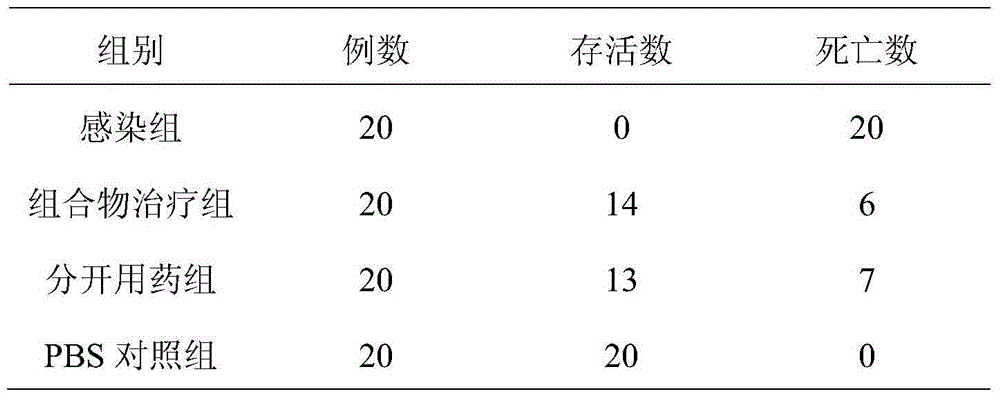
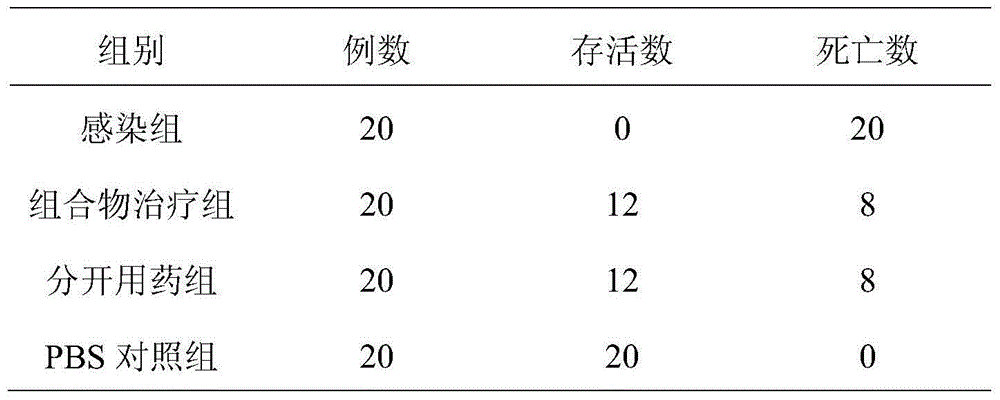
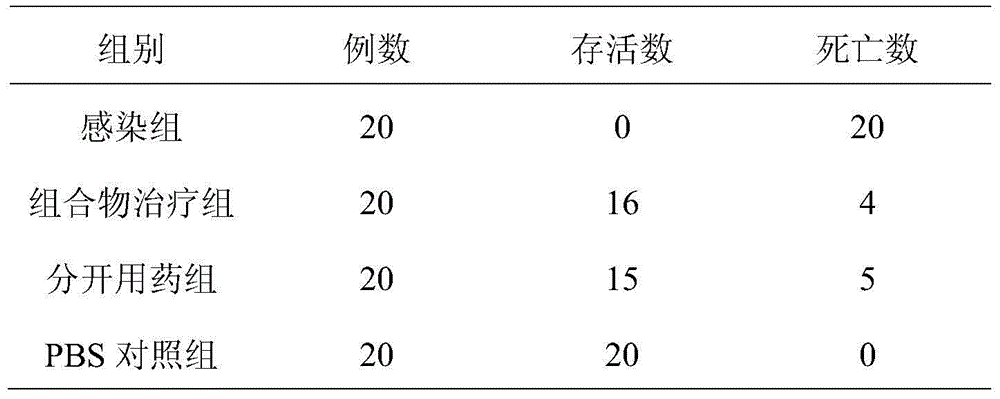
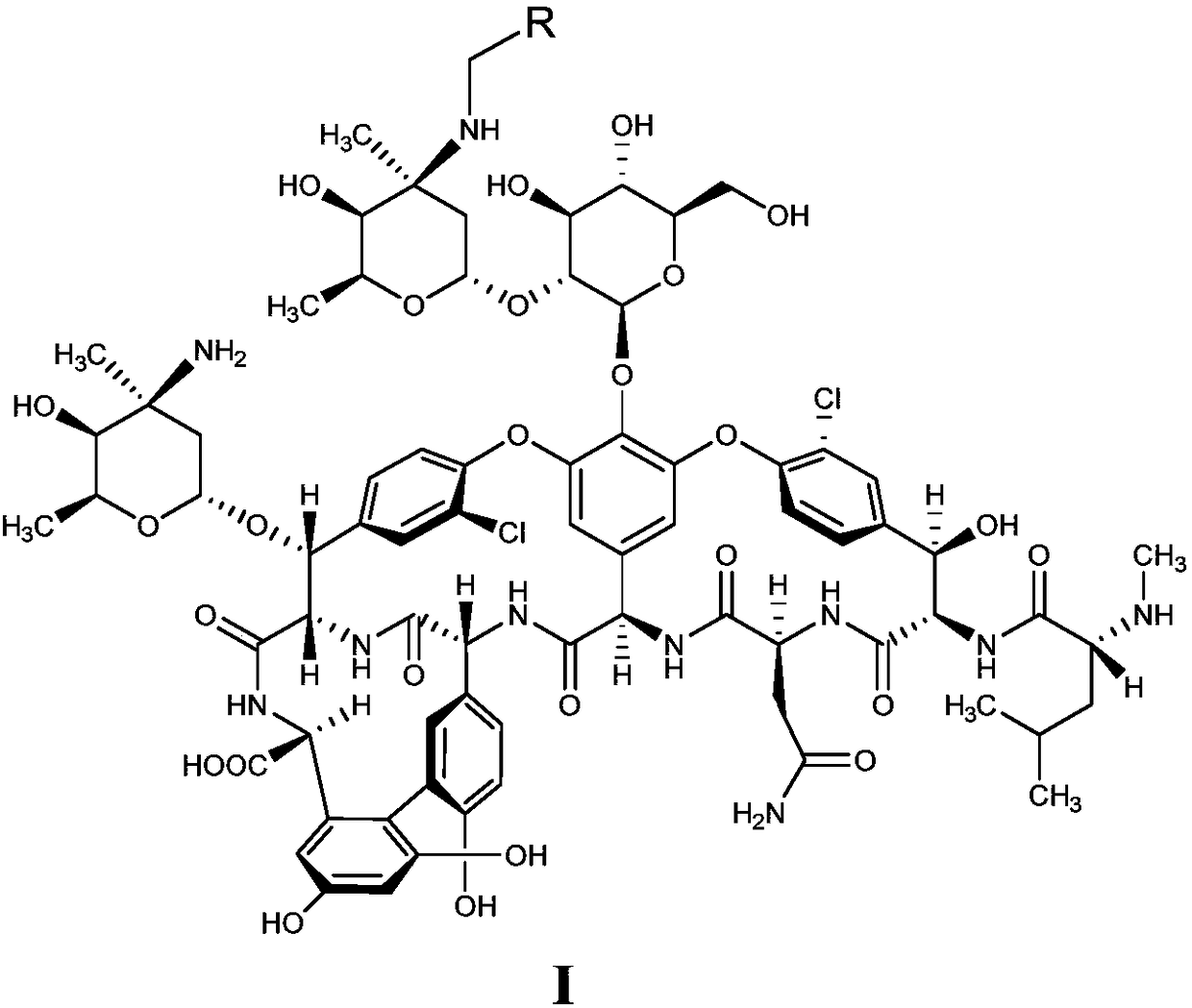
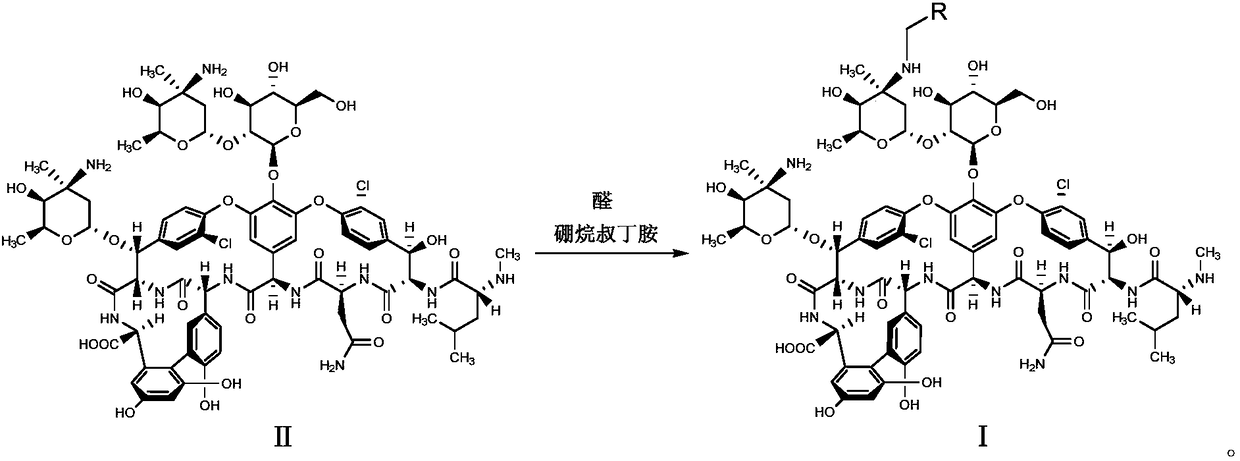
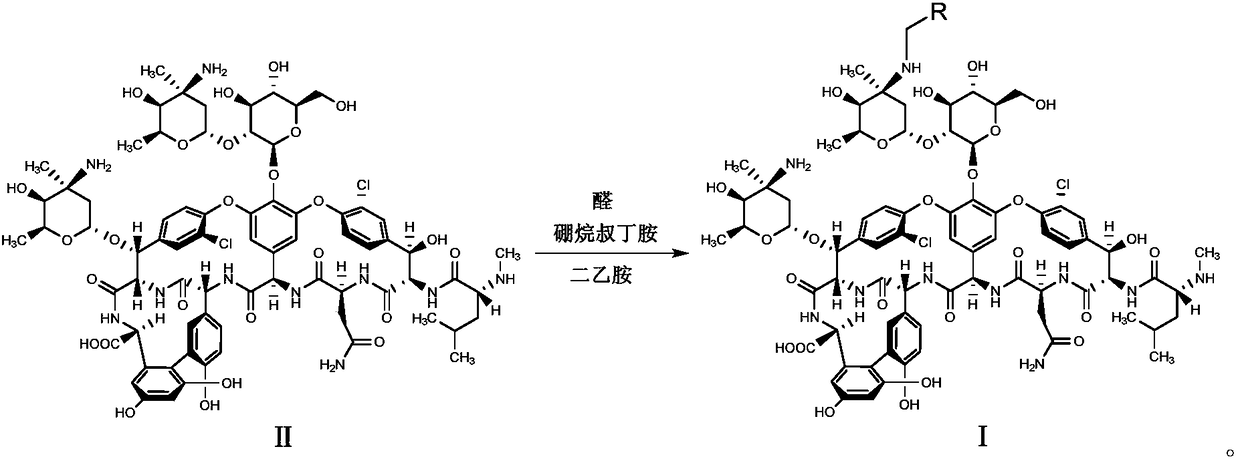
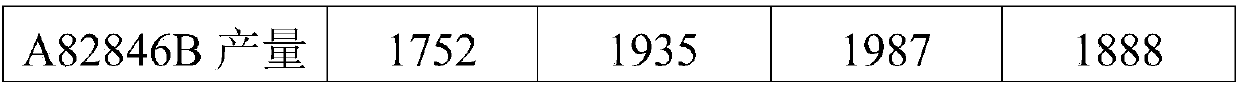Patents
Literature
48 results about "Oritavancin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
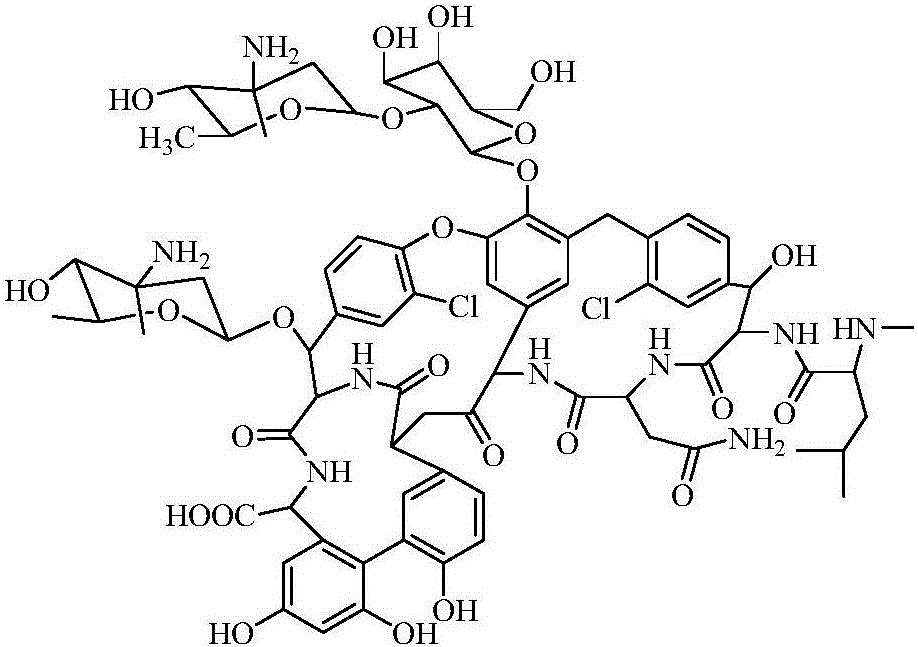
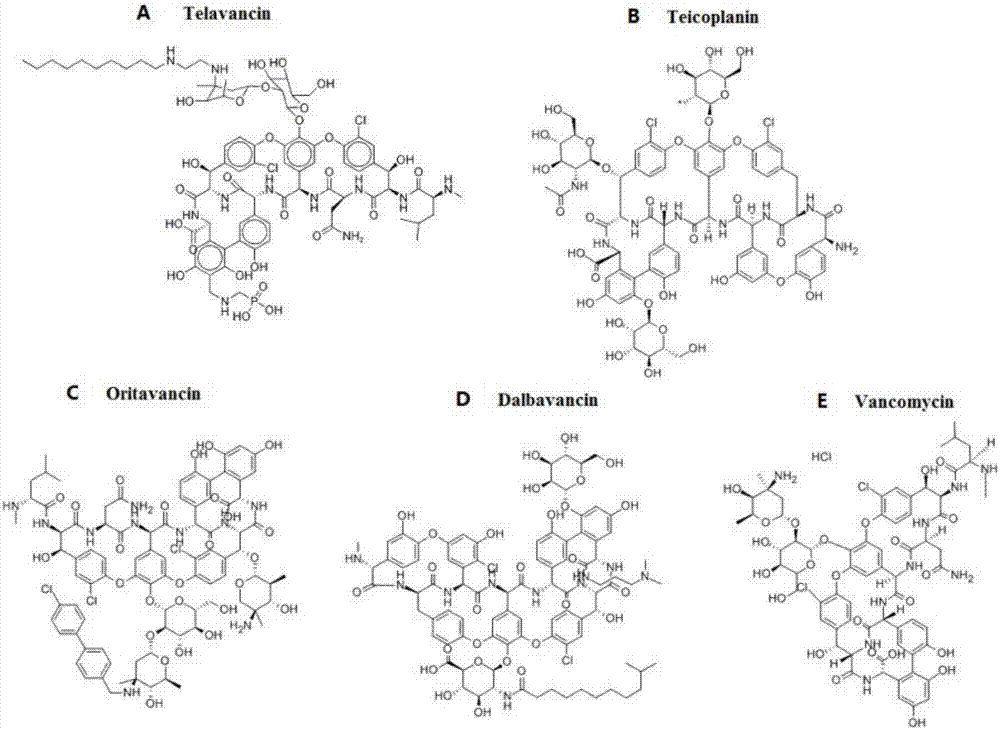
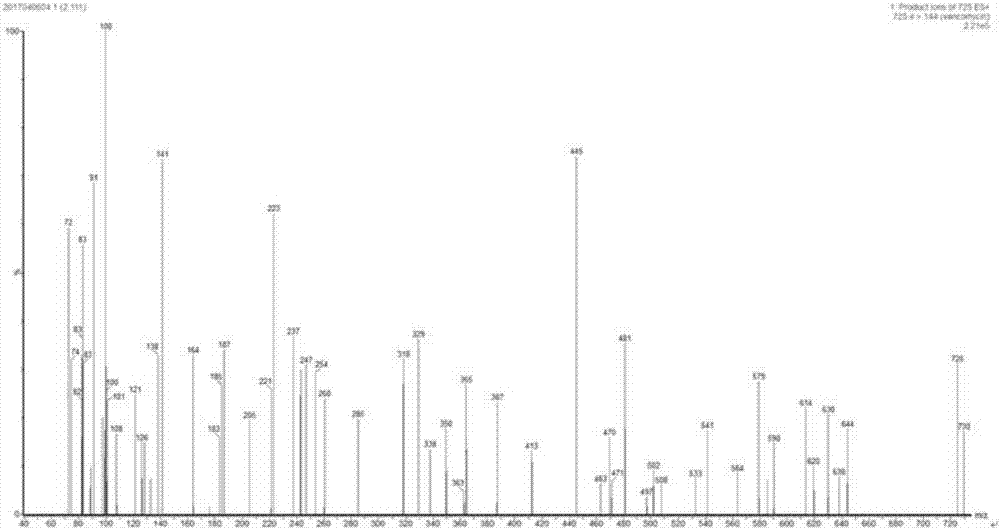
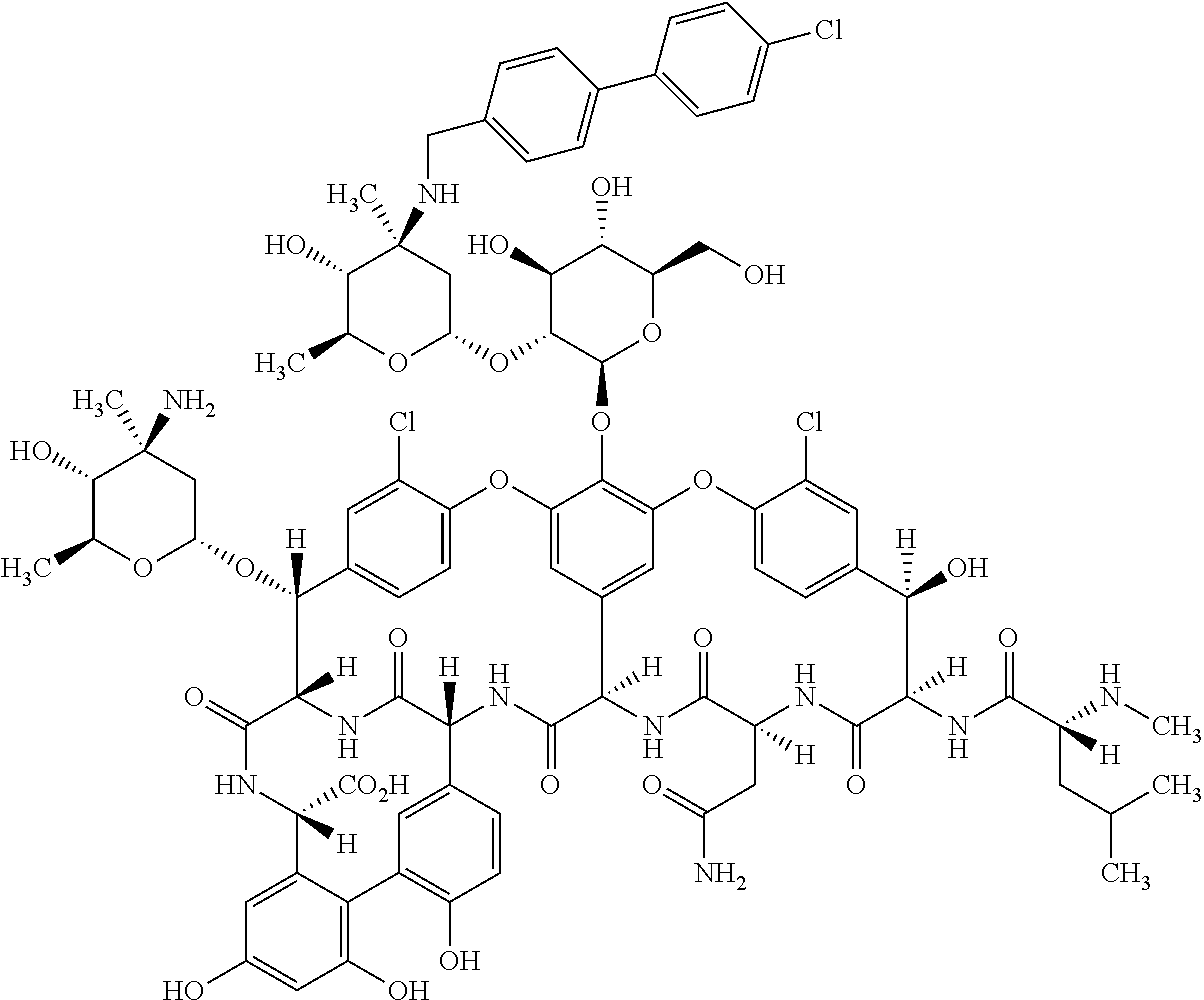
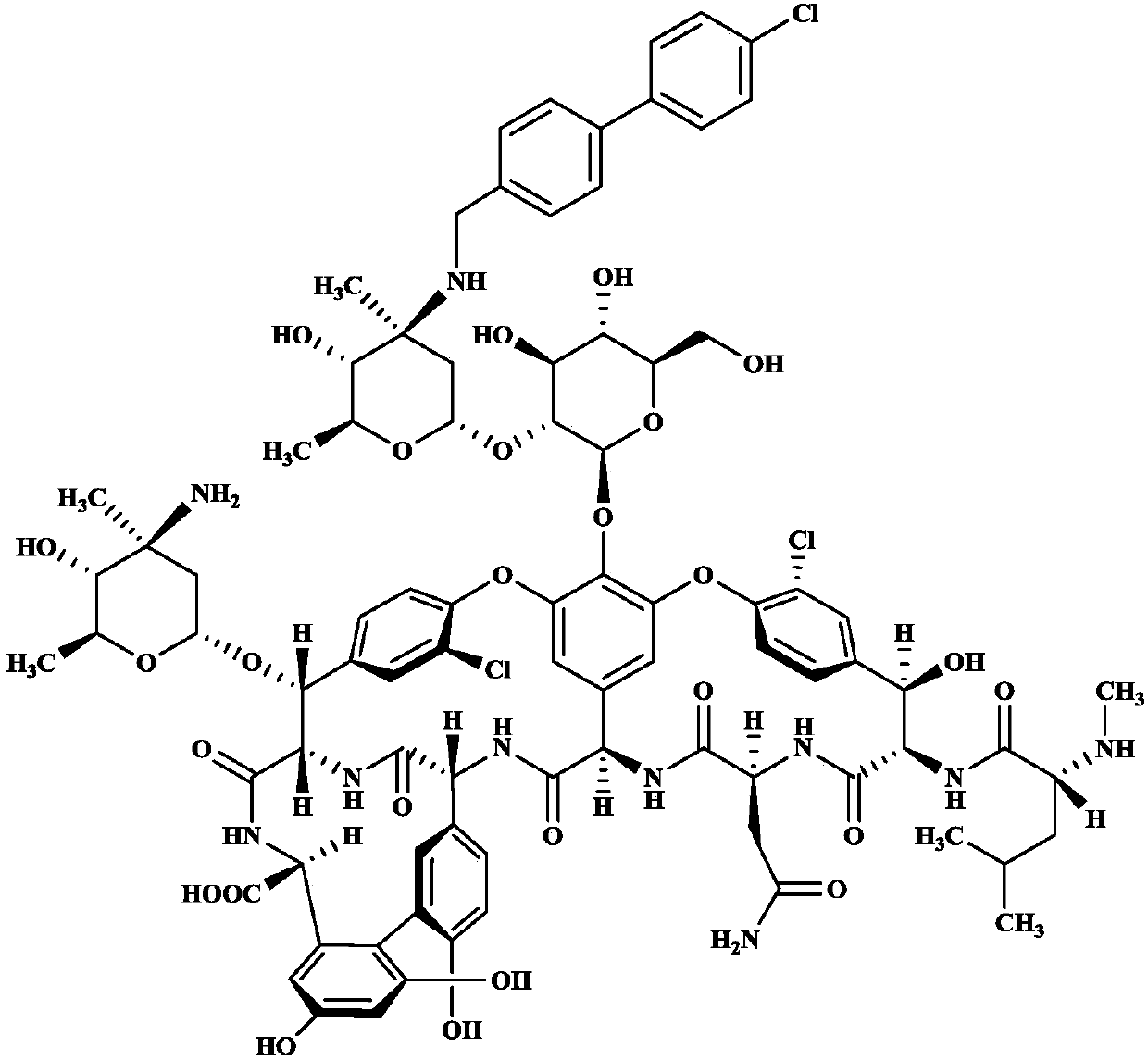
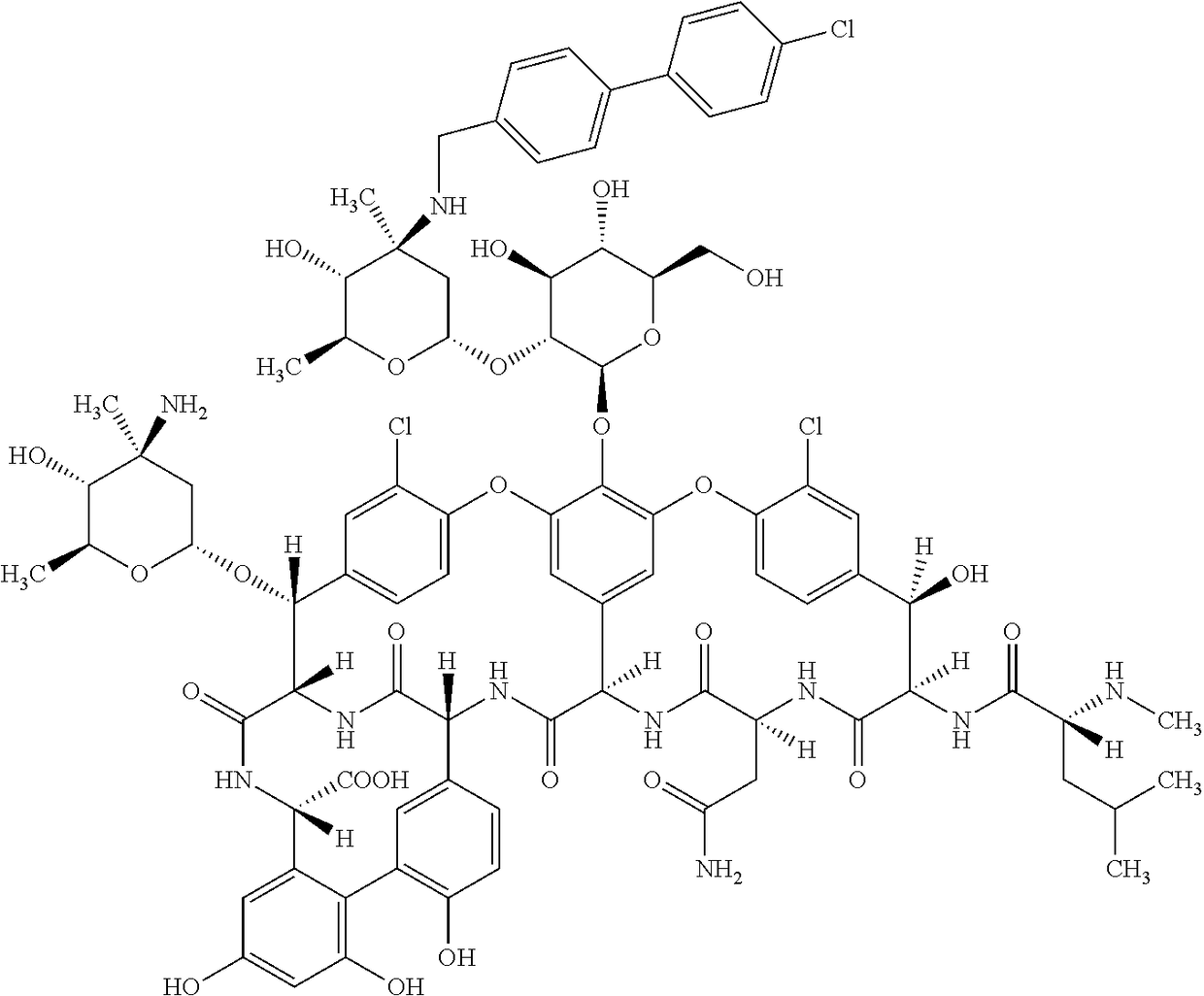
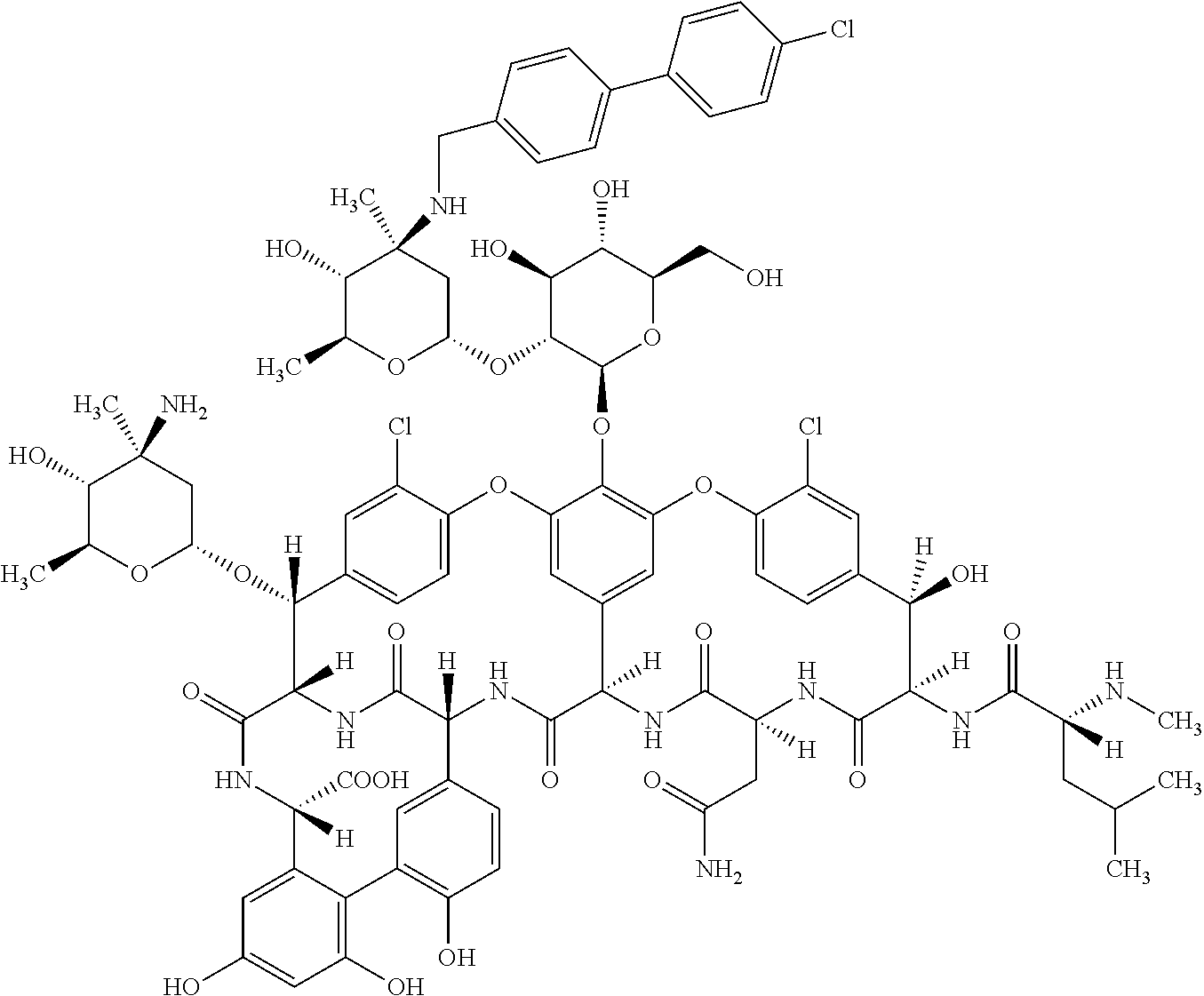
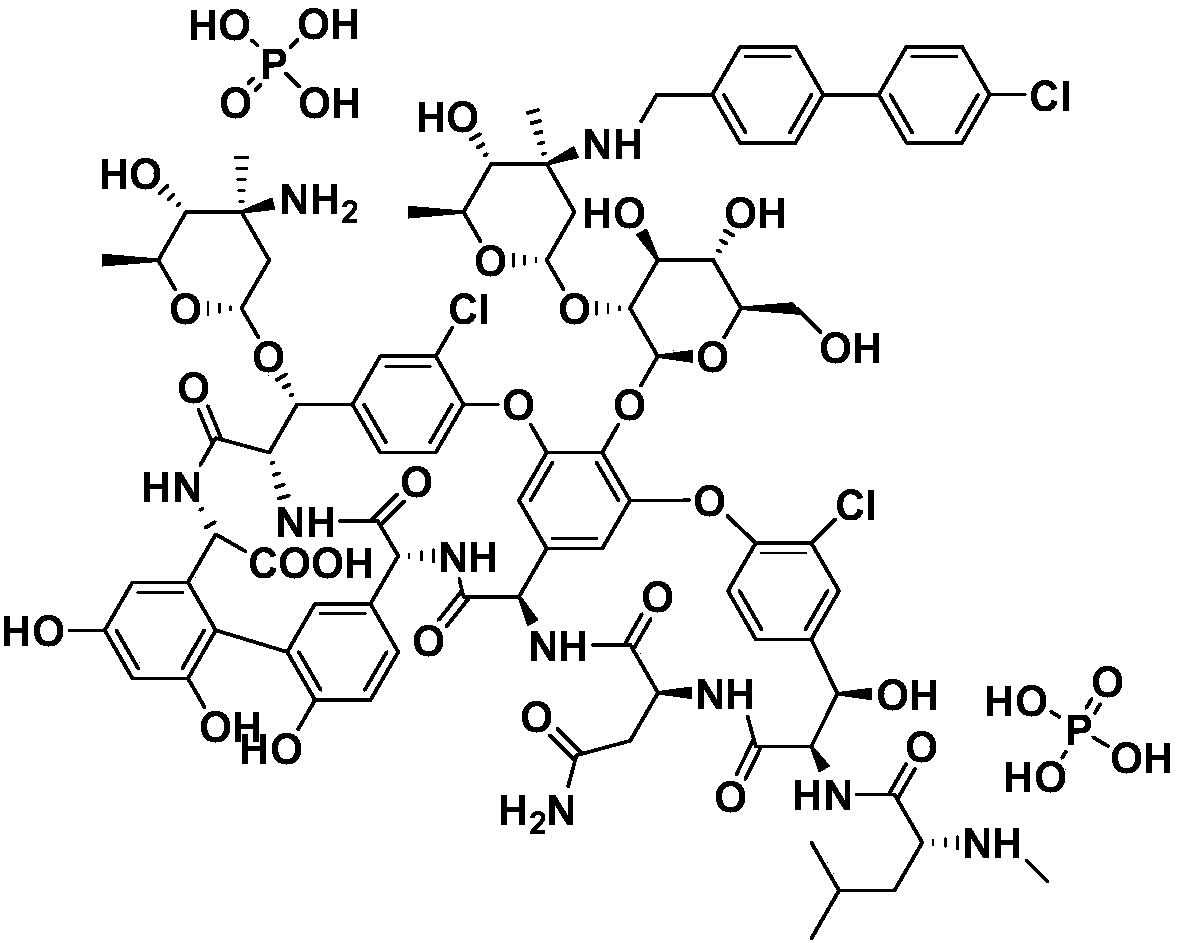
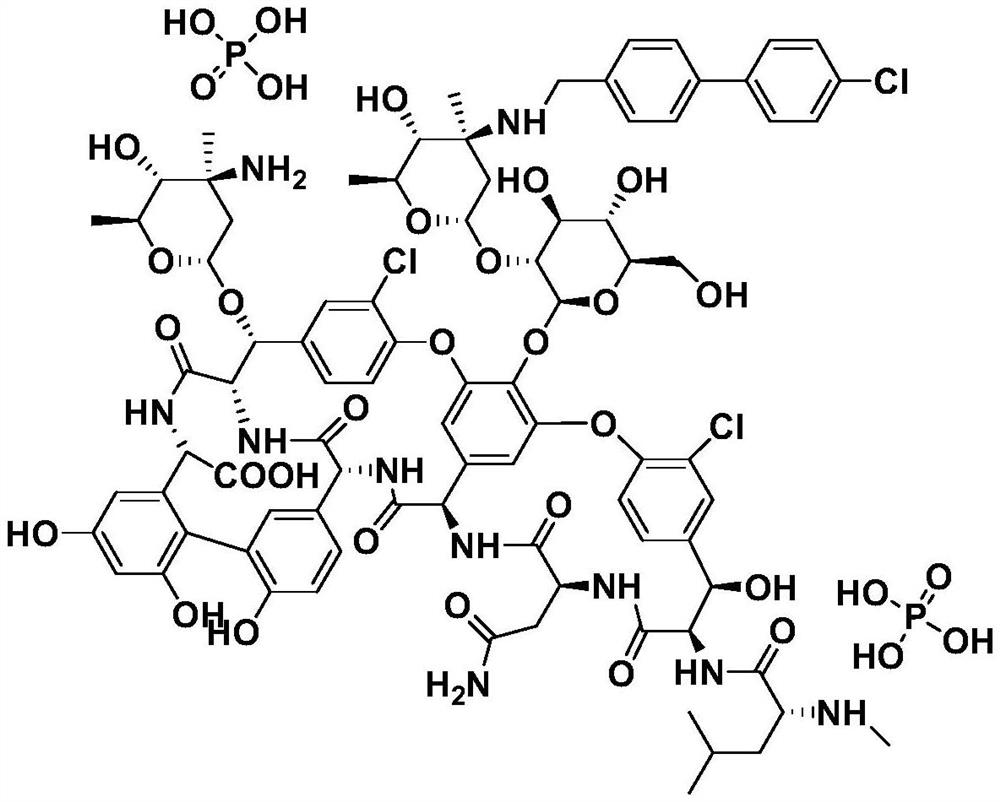
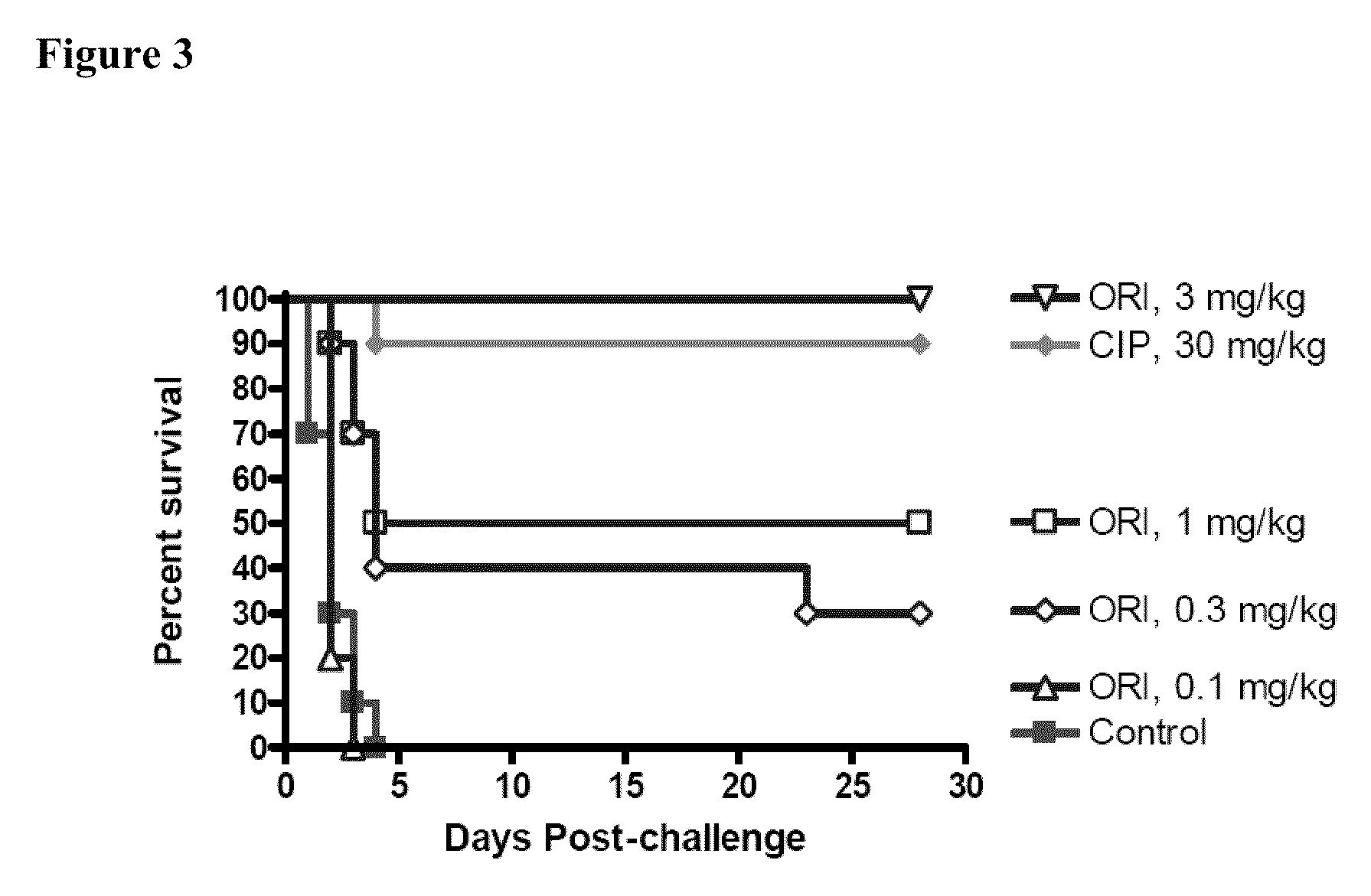
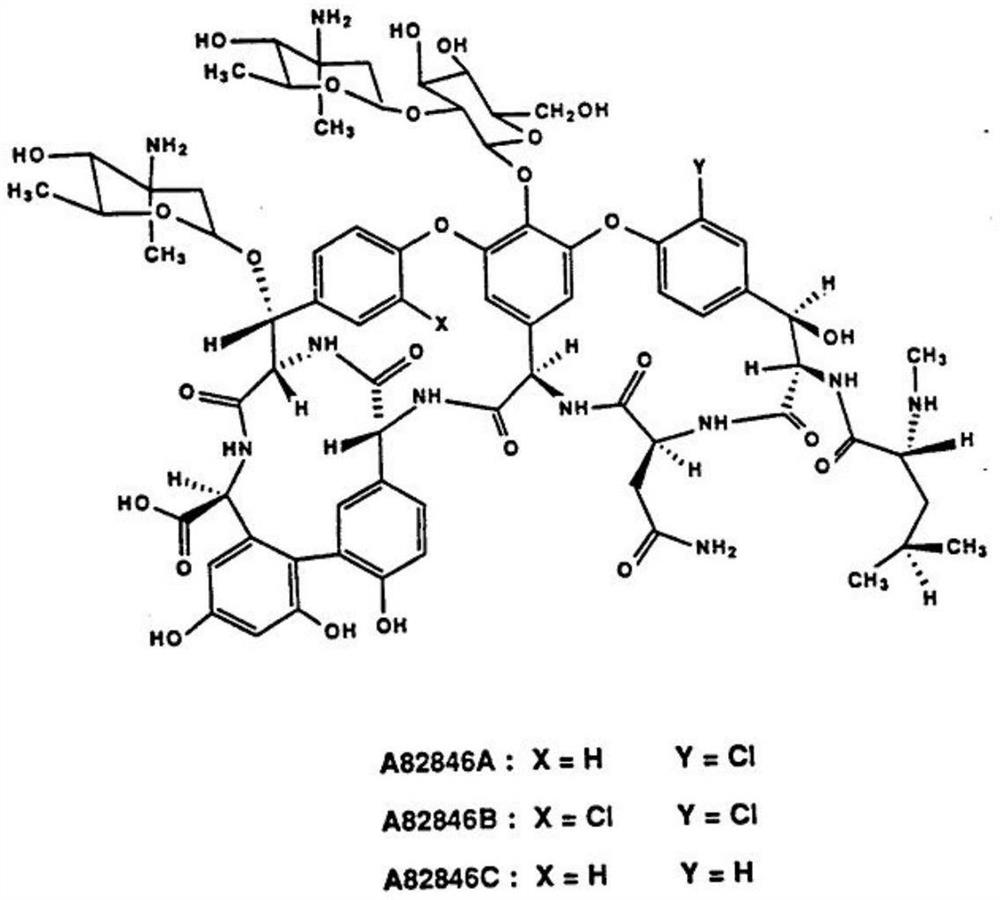
Oritavancin (INN, also known as LY333328, Orbactiv) is a novel semisynthetic glycopeptide antibiotic for the treatment of serious Gram-positive bacterial infections. Its chemical structure as a lipoglycopeptide is similar to vancomycin.
Preparation method of high-purity oritavancin key intermediate A82846B
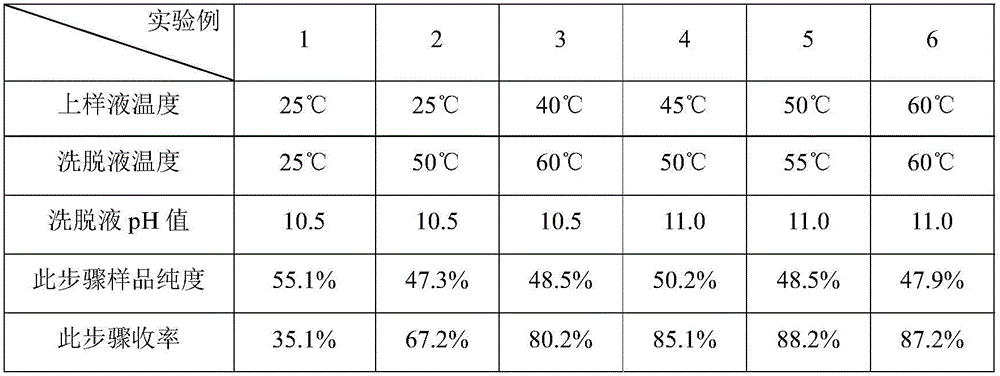
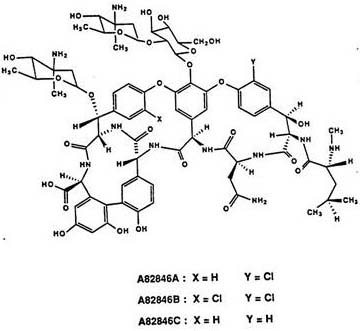
The invention provides a preparation method of high-purity oritavancin key intermediate A82846B. The preparation method comprises the following steps: separating the oritavancin key intermediate A82846B from a fermented solution, first adjusting a pH, purifying by virtue of macroporous resin, then purifying by virtue of inversed phase chromatography, and finally crystallizing to obtain the high-purity A82846B. The method adopted by the invention is simple in operation, less in consumption of organic solvent, and capable of greatly reducing the generation of waste liquid; and moreover, the defects in the prior art that the adsorption efficiency of cation macroporous resin is low and the product yield is reduced due to the leaked adsorption can be overcome, and the preparation method is suitable for the industrialized production.
Owner:CHONGQING QIANTAI BIOLOGICAL MEDICINE
Purification method of Oritavancin intermediate A82846B
ActiveCN107434823AReduce usageReduce energy consumptionPeptide preparation methodsPurification methodsOritavancin
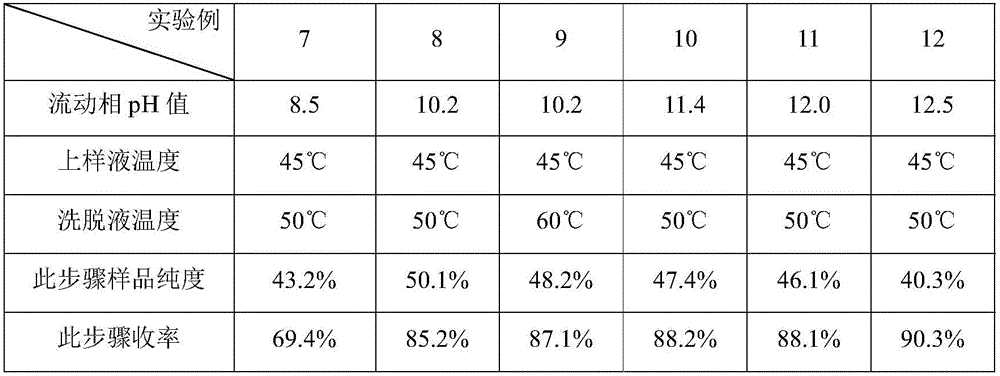
The invention provides a purification method of an Oritavancin intermediate A82846B. Specifically, the method comprises the following steps: loading an A82846B fermentation broth to an ion exchange resin chromatography column, and carrying out elution separation to obtain a first eluate; loading the first eluate toa reversed phase chromatography chromatography column with silica gel C18 or polystyrene polymer as a filler, carrying out impurity elution, carrying out sample elution and collecting a second eluate. Purity of the obtained A82846B is greater than 80%. By the method, quality of the product can be guaranteed; and the method is suitable for an expanded commercial market. The invention also provides a method for preparing the Oritavancin intermediate A82846B salt and a method for synthesizing Oritavancin by the use of the A82846B salt.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Method for simultaneously detecting various glycopeptides antibiotics in animal derived food
ActiveCN107957464AHigh recovery rateThe detection method is sensitive, accurate and fastComponent separationDalbavancinTelavancin
The invention discloses a method for simultaneously detecting various glycopeptides antibiotics in animal derived food. The method mainly comprises the following steps: carrying out solution extraction on a sample; carrying out series purification and enrichment by using HLB solid-phase extraction columns and strong cation (MCX) solid-phase extraction columns; separating the sample by using reversed phase columns, and then detecting the sample by using an ultra-efficient liquid chromatography tandem mass spectrometry in a multi-reaction monitoring mode; and carrying out qualitative and quantitative analysis. Five glycopeptides antibiotics including telavancin, teicoplanin, oritavancin, dalbavancin and vancomycin in the sample animal derived food such as milk, eggs and chicken can be detected simultaneously. The detecting method is sensitive, accurate and rapid; and the five glycopeptides antibiotics can be qualitatively and quantitatively analyzed by six minutes displayed on an instrument. Moreover, the detecting method also has certain reference value for detection of other glycopeptides antibiotics.
Owner:GUANGZHOU CENT FOR DISEASE CONTROL & PREVENTION (GUANGZHOU HYGIENE INSPECTION CENT GUANGZHOU CENT FOR FOOD SAFETY RISK SURVEILLANCE & ASSESSMENT INST OF PUBLIC HEALTH OF GUANGZHOU MEDICAL UNIV)
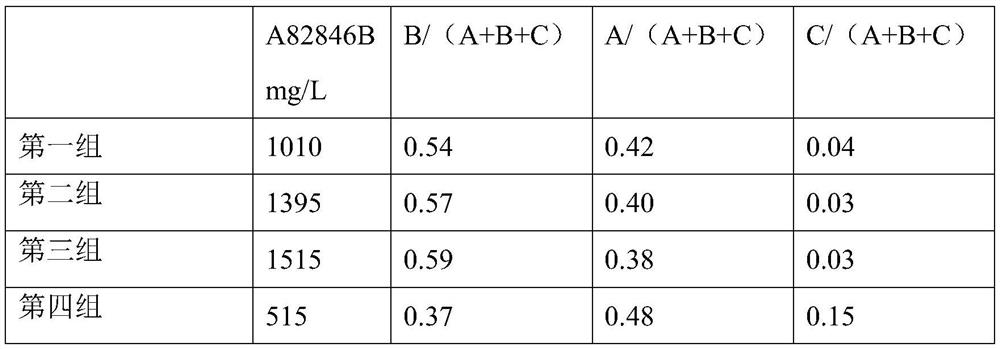
Fermentation medium and production process for producing oritavancin precursor A82846B
ActiveCN107557415AIncrease productionReduce the ratioMicroorganism based processesFermentationYeastOritavancin
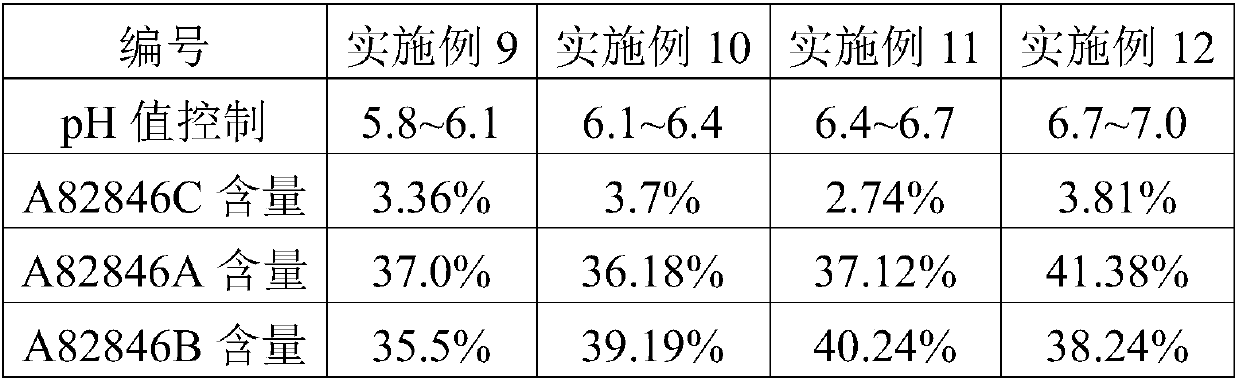
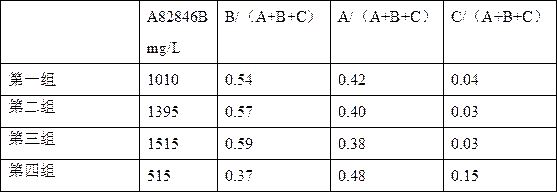
The invention discloses a fermentation medium and a production process for producing an oritavancin precursor A82846B. The fermentation medium is used for producing the oritavancin precursor A82846B,and the fermentation medium consists of an organic carbon source, an organic nitrogen source, mineral substances and water, wherein the organic carbon source includes 5-10% of glucose in terms of themass percentage of the fermentation medium, and the organic nitrogen source includes 0.3-1.0% of yeast powder in terms of the mass percentage of the fermentation medium. With the application of the fermentation medium provided by the invention, the yield of the A82846B is greatly improved, and the proportions of two impurities, namely A82846A and A82846C, are obviously reduced. The production process for producing the oritavancin precursor A82846B provided by the invention can effectively improve the yield of the A82846B and reduce the content of the impurities; the production process is applicable to production by virtue of fermenters; and efficient production of the oritavancin precursor A82846B can be achieved.
Owner:SHANGHAI INST OF PHARMA IND +1
Method for preparing oritavancin
The invention discloses an improved method for preparing oritavancin. The method takes A82846B acetate as a raw material, under existence of a Brettphos catalyst, a Brettphos ligand, alkali and a solvent, the material and an amino alkylation reagent are subjected to a reaction to generate oritavancin with one step. According to the invention, reaction steps are simplified, the selectivity of amino alkylation is increased, oritavancin yield is increased, and a route suitable for large industrial production is provided.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
Use of oritavancin for prevention and treatment of anthrax
ActiveUS20100041585A1Inhibition of colonizationAntibacterial agentsOrganic active ingredientsDiseaseOritavancin
Glycopeptide antibiotics, such as oritavancin, demonstrate significant activity against B. anthracis. Methods for the treatment, prophylaxis and prevention of B. anthracis infection and disease in animals, including humans, are described.
Owner:MELINTA THERAPEUTICS
Medicinal composition for treating complex infection and preparation method of medicinal composition
InactiveCN104888222AConvenient clinical administrationAntibacterial agentsOrganic active ingredientsEchinocandinOritavancin
The invention relates to a medicinal composition for treating complex infection and a preparation method thereof. The medicinal composition is characterized by comprising an active ingredient selected from one of glycopeptides antibacterial drugs of telavancin, dalbavancin, oritavancin, vancomycin, teicoplanin or a pharmaceutically acceptable salt thereof, and also comprising polyene and echinocandin type antifungal agents, and being prepared into a freeze-dried powder injection by adopting a conventional process. The medicinal composition provided by the invention realizes combined application of the antibacterial drugs and the antifungal agents, can simultaneously give play to an antibacterial infection effect and an antifungal infection effect, and is convenient for clinical use.
Owner:SHENZHEN JYMED TECH
Glycopeptides compounds with anti-resistance bacterial activity, preparation method and application thereof
ActiveCN108409837AImprove securityStrong inhibitory activityAntibacterial agentsInorganic non-active ingredientsDiseaseOritavancin
The invention discloses a group of glycopeptides compounds with anti-resistance bacterial activity. The glycopeptides compounds in accordance with a general formula (I) are as shown in the following specification. The invention further provides a preparation method and an application for the above glycopeptides compounds. Through testing, compared with a second-generation glycopeptides pharmaceutical oritavancin, the glycopeptides antibiotic compounds have the higher inhibitory activity to endurance strains, especially MRSA or VRE. Through further testing, the most of the glycopeptides compounds have the higher safety than the oritavancin, and can be used for preparing pharmaceuticals for treating or preventing various diseases, such as skin and soft tissue infection, cephalomeningitis, sepsis, pneumonia, arthritis, peritonitis, bronchitis, and empyema, caused by bacterial infection.
Owner:SHANGHAI LAIYI BIOMEDICAL RES & DEV CENT +1
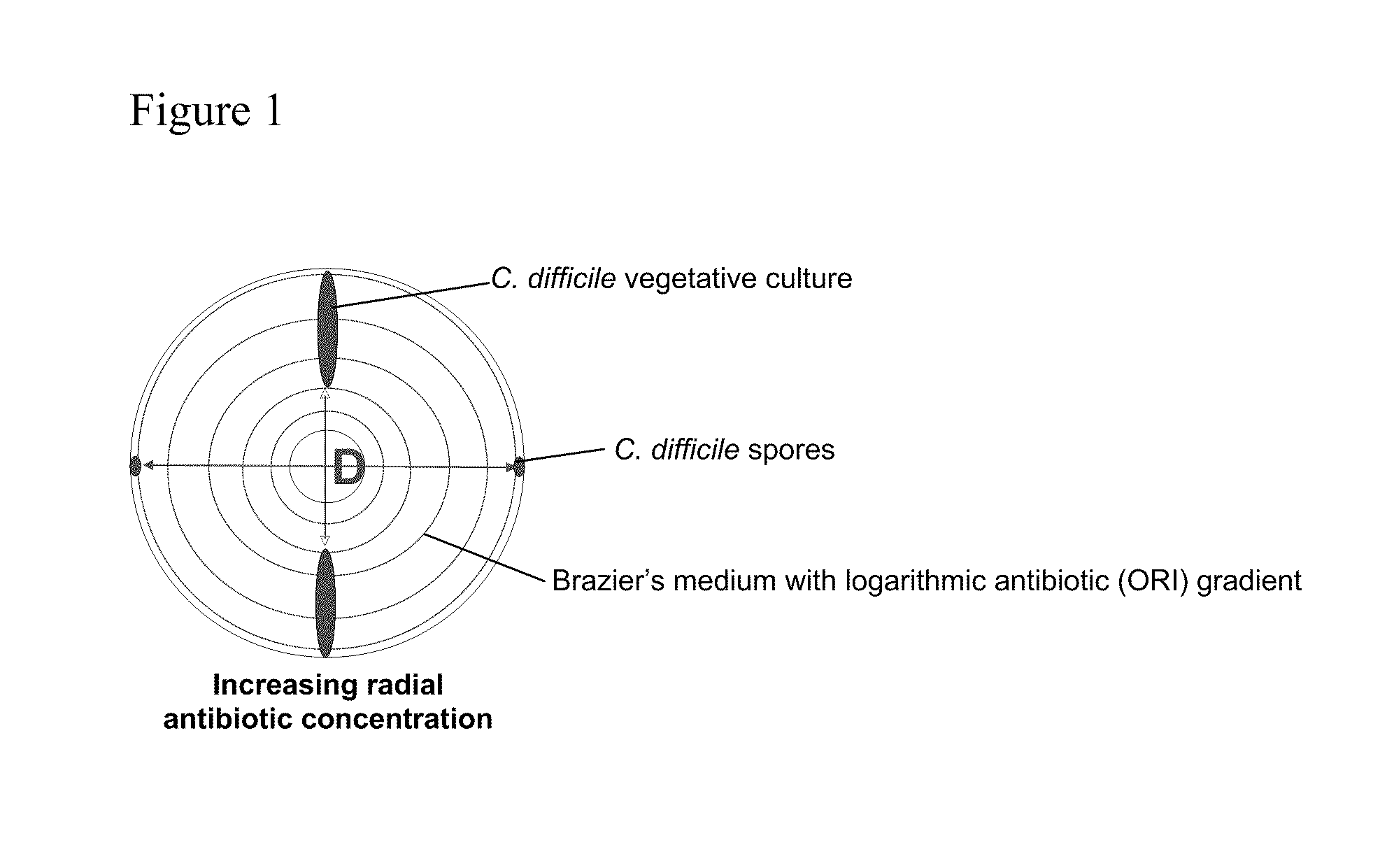
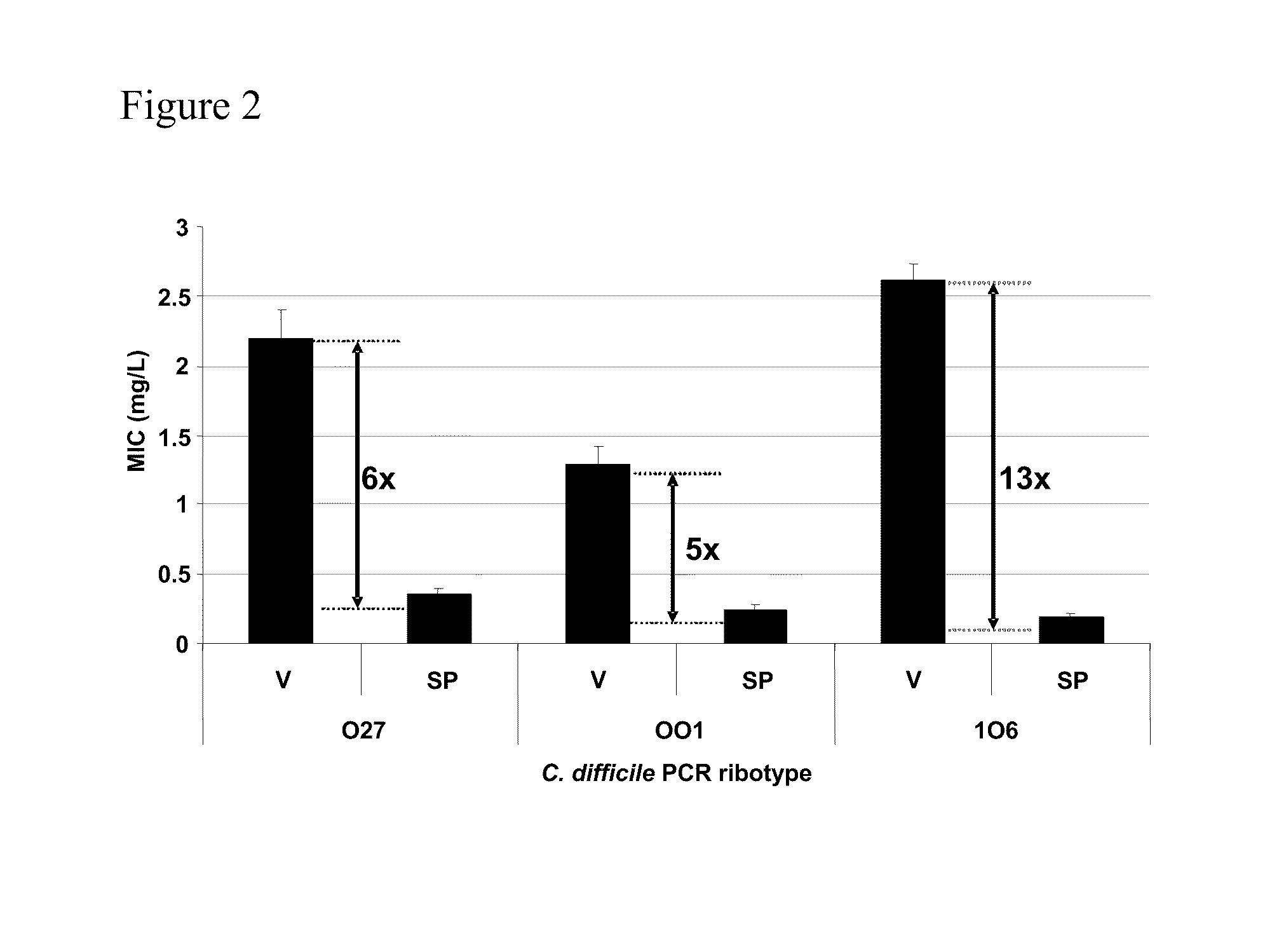
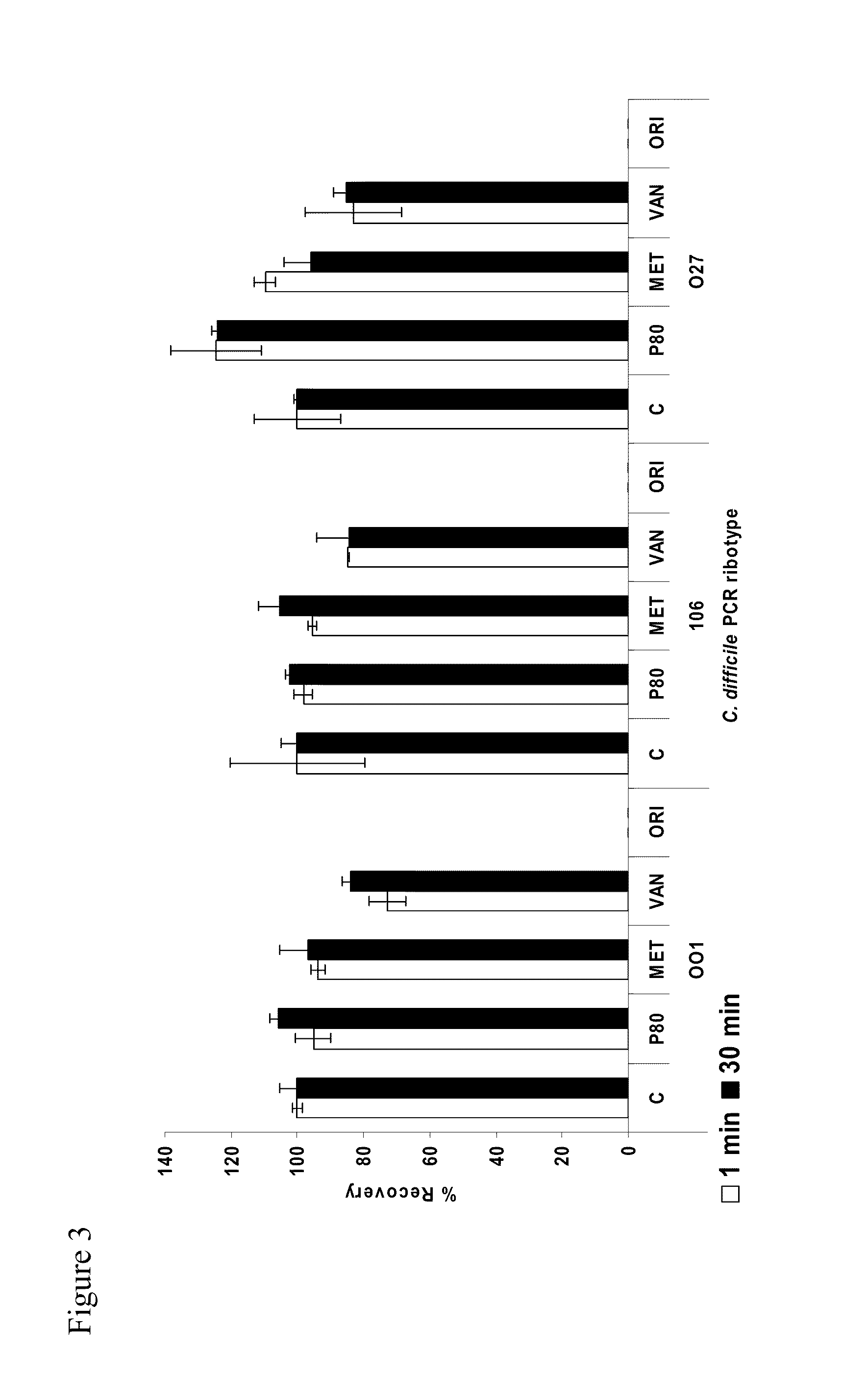
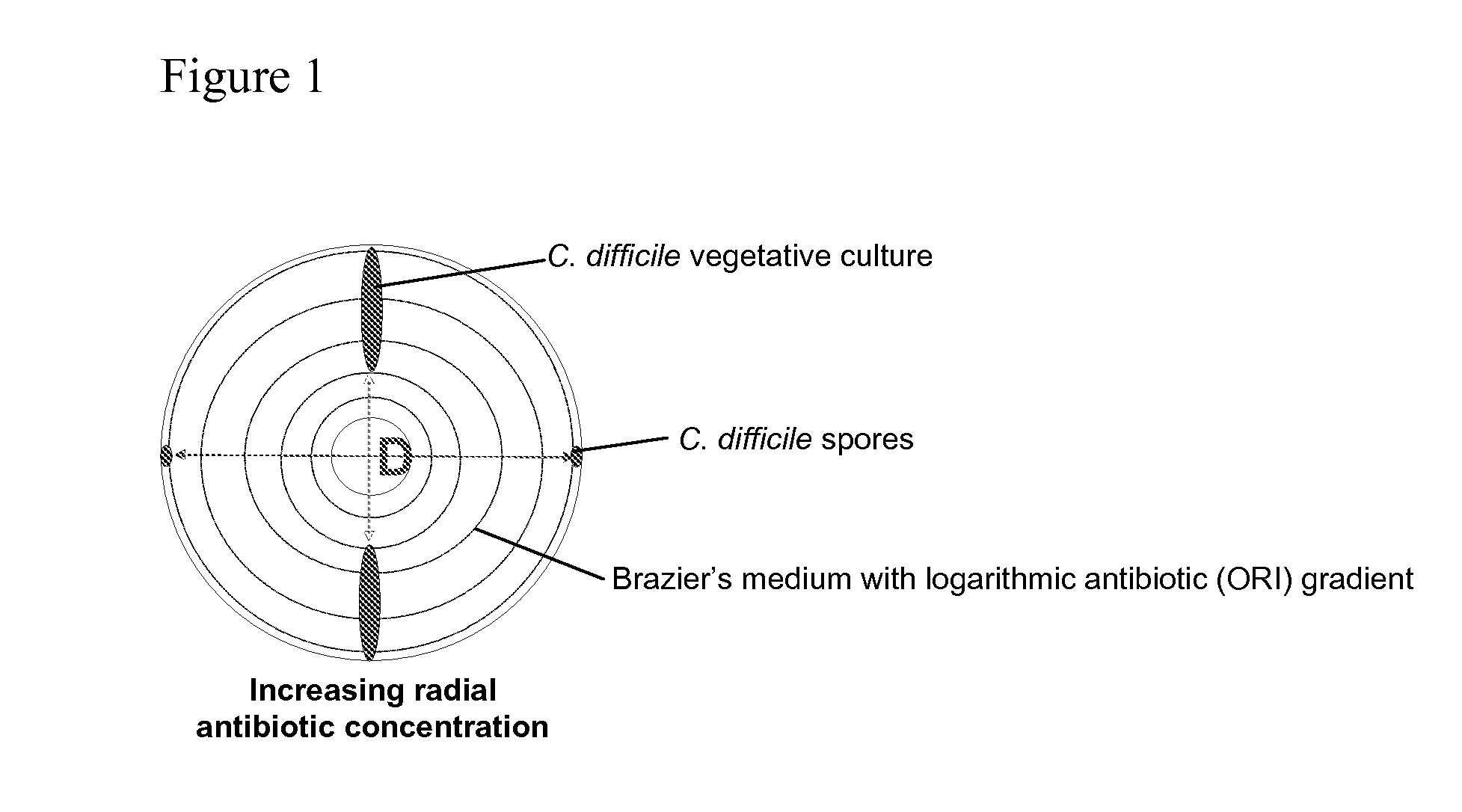
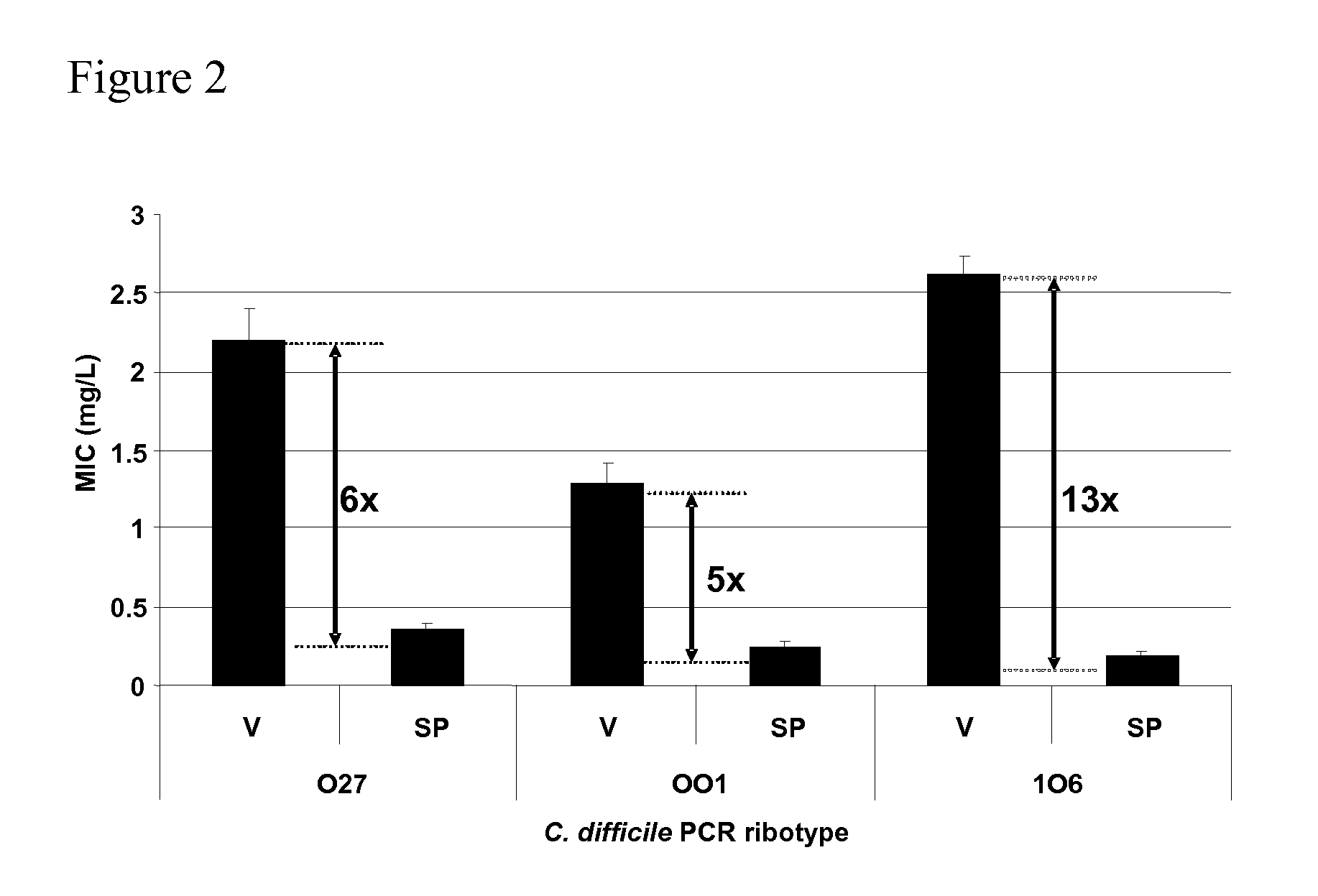
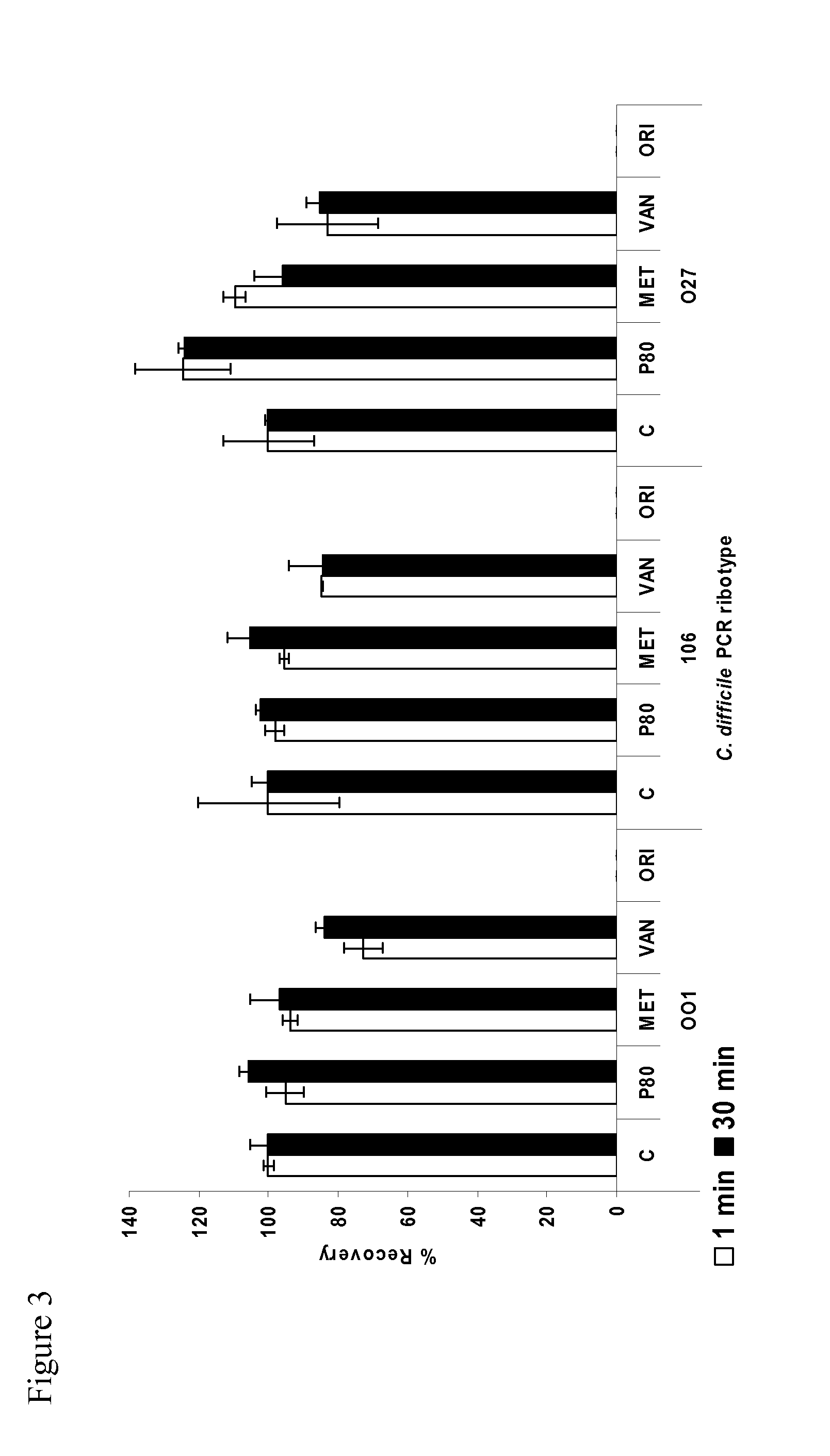
Method of inhibiting clostridium difficile by administration of oritavancin
Glycopeptide antibiotics, such as oritavancin, demonstrate significant activity against both a vegetative form of C. difficile and C. difficile spores. Methods for the treatment, prophylaxis and prevention of C. difficile infection and disease in animals, including humans, are described.
Owner:MELINTA THERAPEUTICS
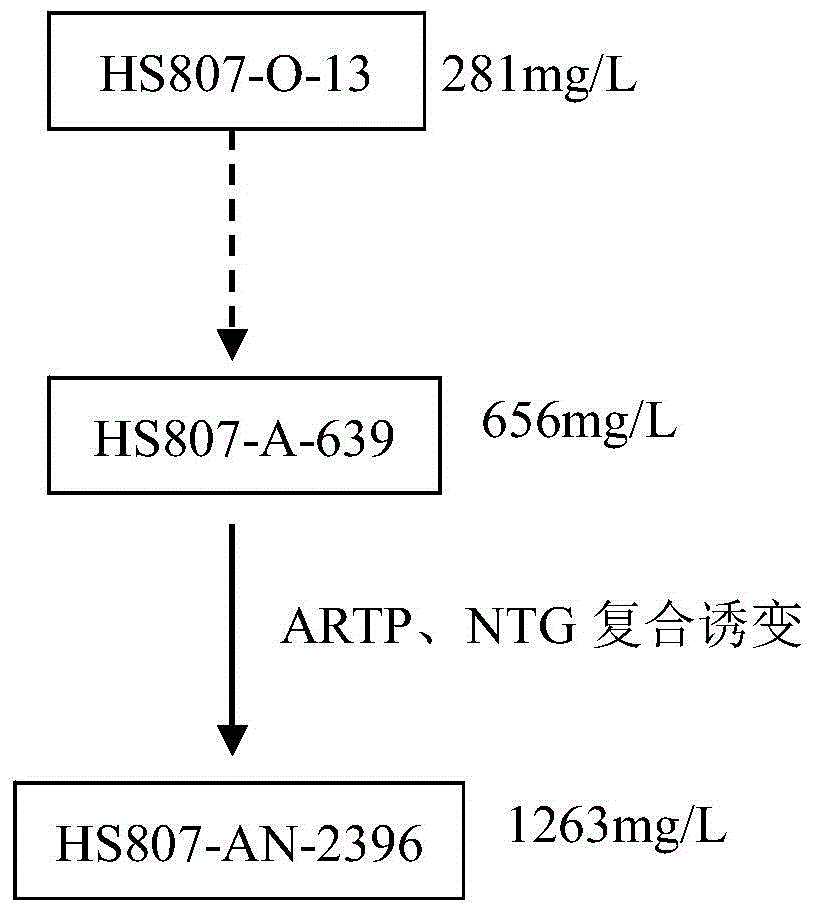
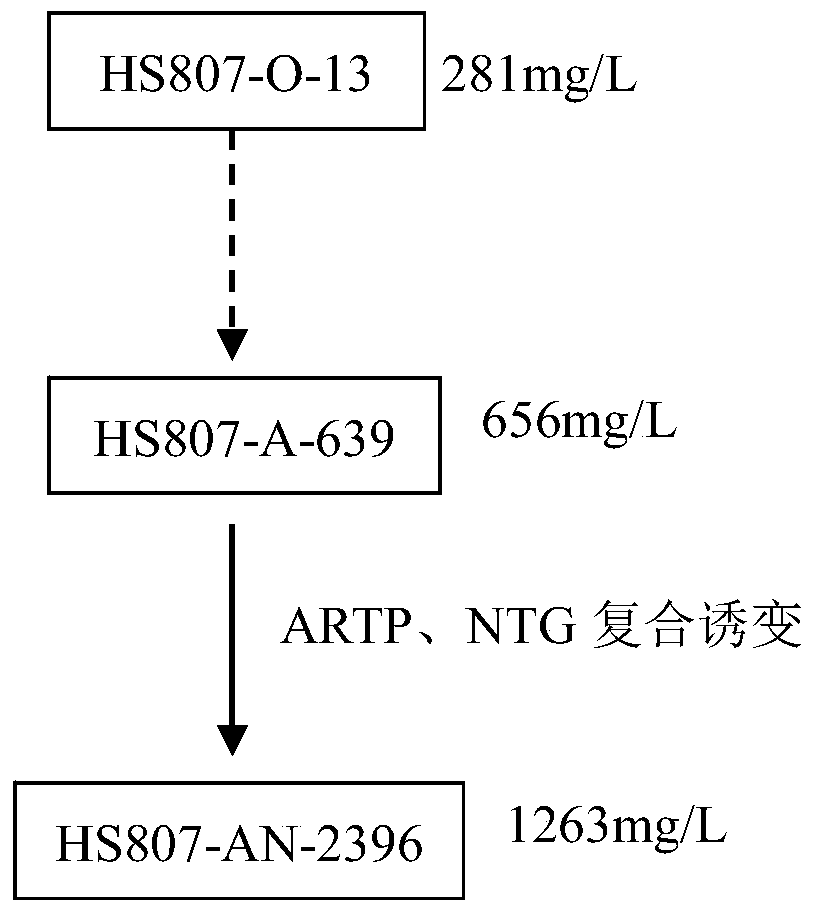
Kibdelosporangium aridum and preparation method of oritavancin intermediate
ActiveCN106190854AEase of industrial productionIncrease productionFungiMicroorganism based processesMicroorganismOritavancin
The invention discloses kibdelosporangium aridum and a preparation method of an oritavancin intermediate. The kibdelosporangium aridum strain HS807-AN-2396 with high yield of the oritavancin intermediate is preserved in the China General Microbiological Culture Collection Center (CGMCC), and is assigned with the accession number of CGMCC No.10576. The preparation method of the oritavancin intermediate comprises the following step: fermenting the kibdelosporangium aridum CGMCC No.10576 in a fermentation medium, thus obtaining the oritavancin intermediate A82846B from the fermentation broth. With the adoption of the preparation method, the high yield, namely, 2315mg / L of the fermentation potency of the oritavancin intermediate can be realized on a 60m<3> tank, and thus the industrial production of oritavancin is facilitated.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Oritavancin formulations
Disclosed herein are antibacterial compositions, pharmaceutical compositions, and the use and preparation thereof. Some embodiments relate to compositions including oritavancin and their use as therapeutic agents.
Owner:THE MEDICINES
Stable oritavancin compound
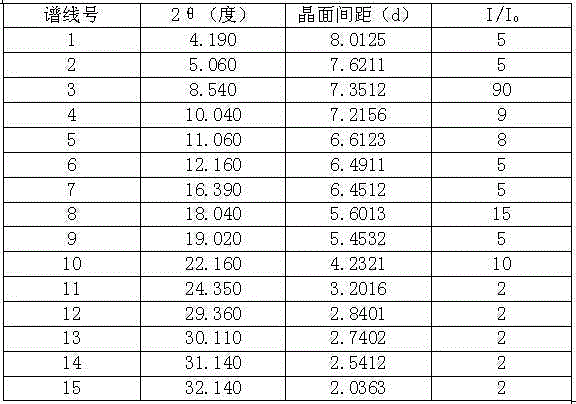
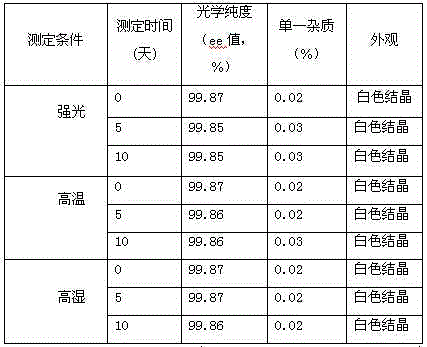
The present invention belongs to the technical field of medicine, and particularly relates to an oritavancin compound and a preparation method thereof. The oritavancin compound of the present invention has advantages of high purity, good stability, and not significant moisture absorption weight increase under a high humidity condition.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Treatment and prevention of bacterial skin infections using oritavancin
Methods for the treatment and prevention of bacterial skin infections using the glycopeptide antibiotic oritavancin are disclosed.
Owner:MELINTA THERAPEUTICS
Oritavancin purification method
PendingCN109988226AHigh purityLow impurity contentPeptide preparation methodsPurification methodsFreeze-drying
The invention provides an oritavancin purification method. The oritavancin purification method includes the following steps: step (1), dissolving crude oritavancin in an aqueous solution of acetonitrile, performing column chromatography by a polymer microsphere chromatography column, and collecting a chromatographic solution having the oritavancin content higher than 98.5%; step (2), removing solvents from the collected chromatographic solution, and performing concentration to obtain an oritavancin concentrate; step (3), performing filtering and freeze-drying to obtain oritavancin powder. Theoritavancin purification method has the advantages that high-purity oritavancin is obtained through polymer microsphere chromatography, and accordingly, the content of impurities is greatly reduced; the content of the oritavancin can reach more than 98.5% through one-step chromatography, and the method is simple; phosphate and methanol / ethanol / acetonitrile serve as mobile phases for chromatography, later treatment and solvent recovery are facilitated, concentration can be conducted through nanofiltration membranes, and the method is convenient to operate; the purification method improves the product quality and is suitable for expanded commercial production.
Owner:SHANGHAI LAIYI BIOMEDICAL RES & DEV CENT
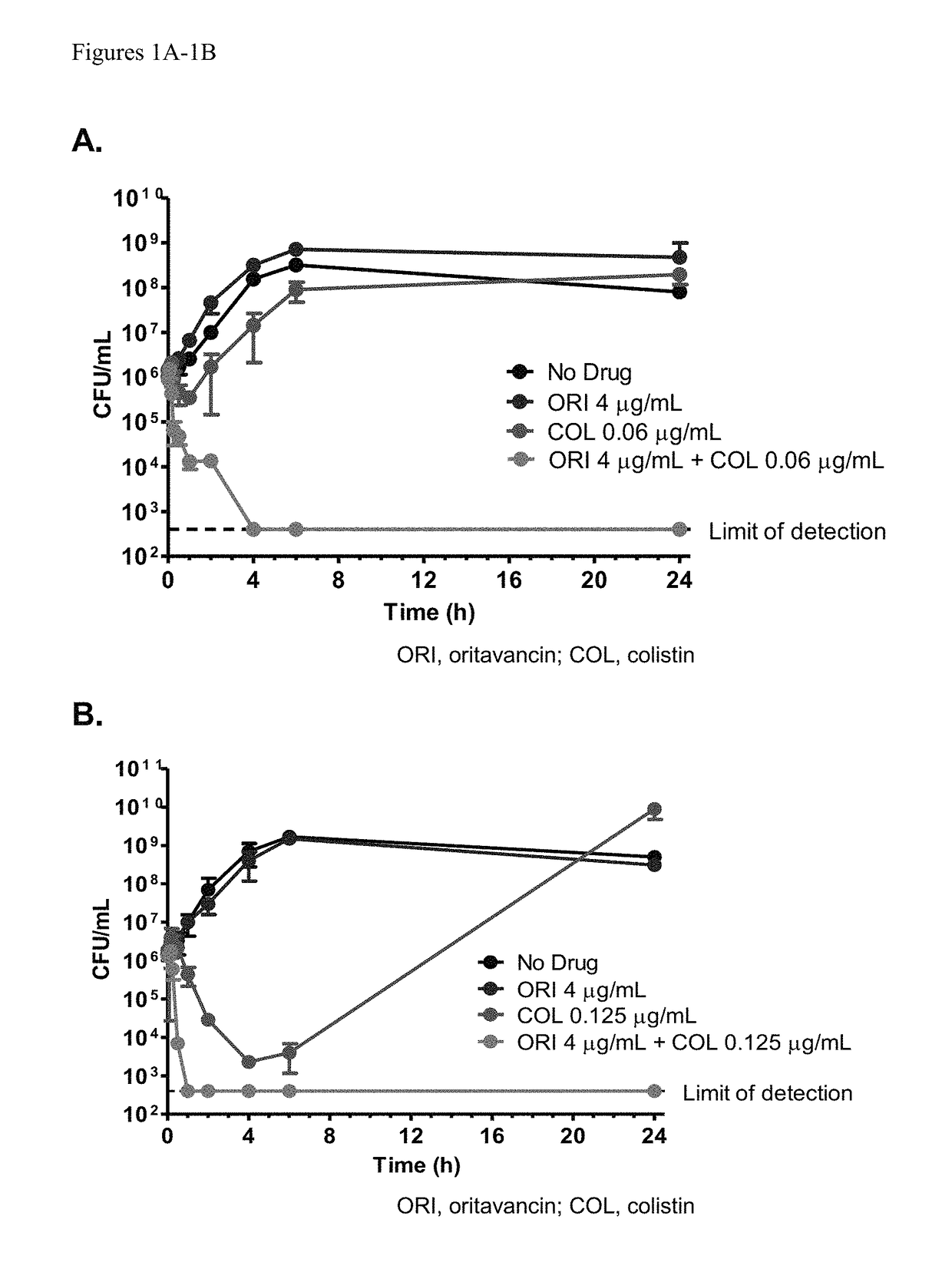
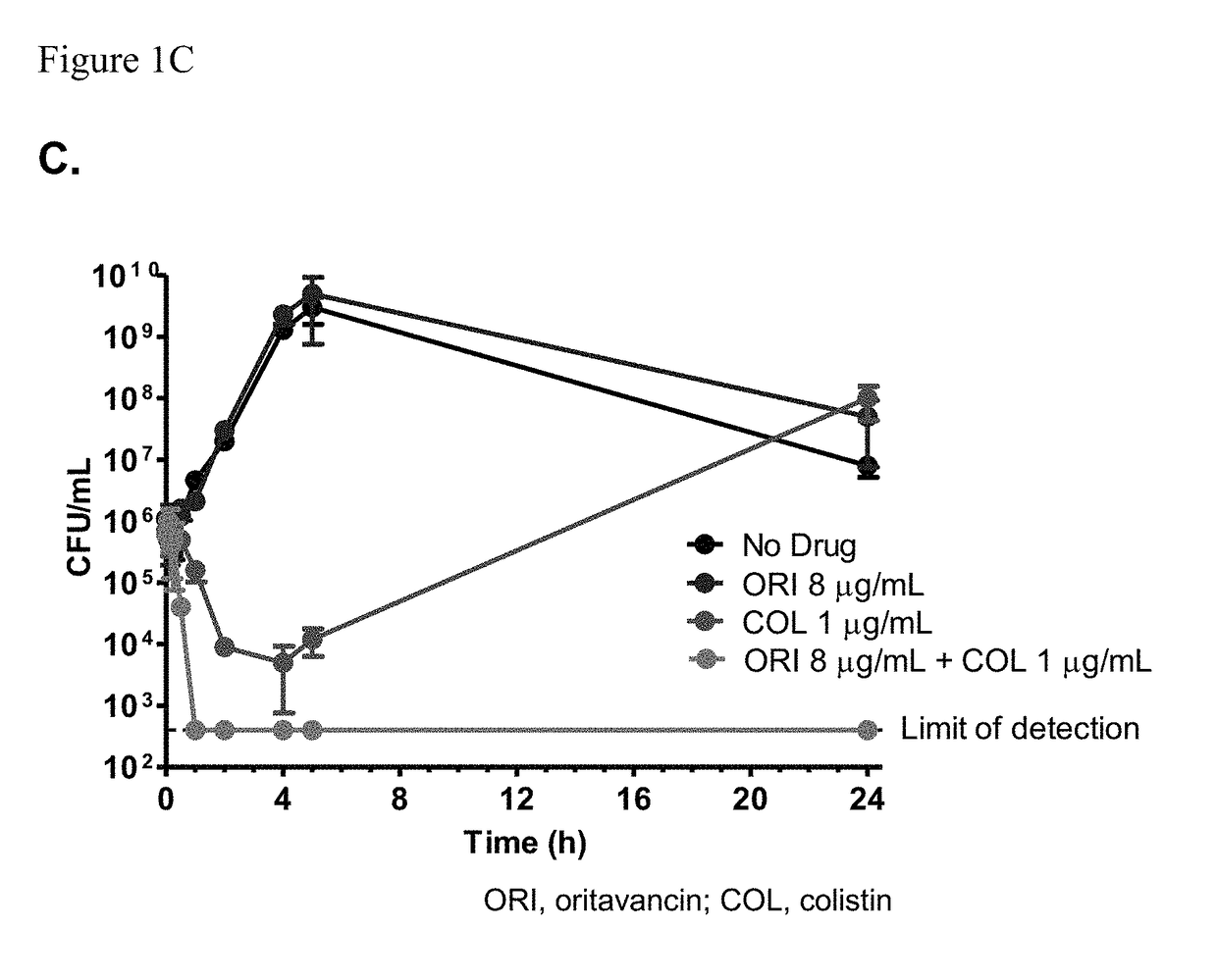
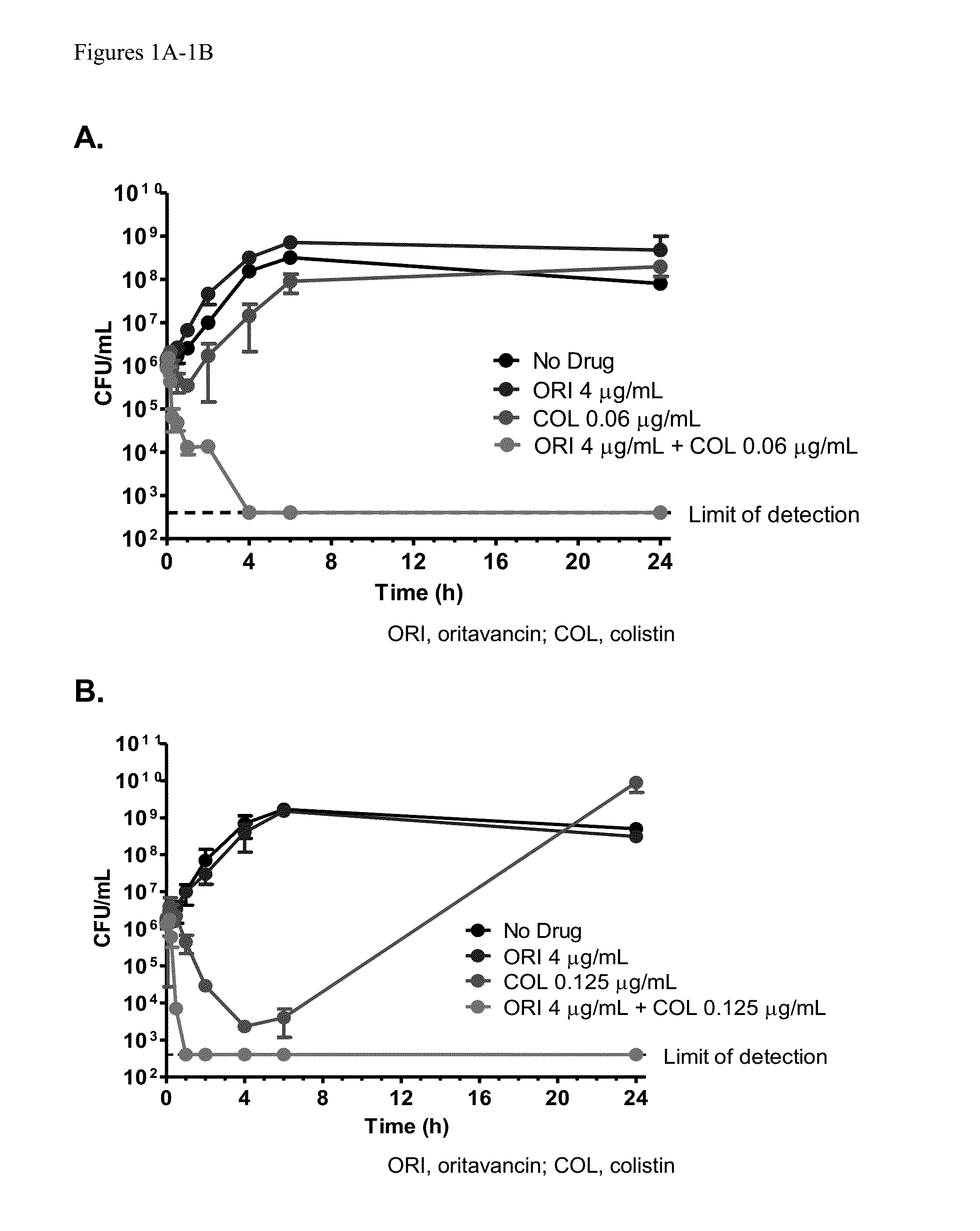
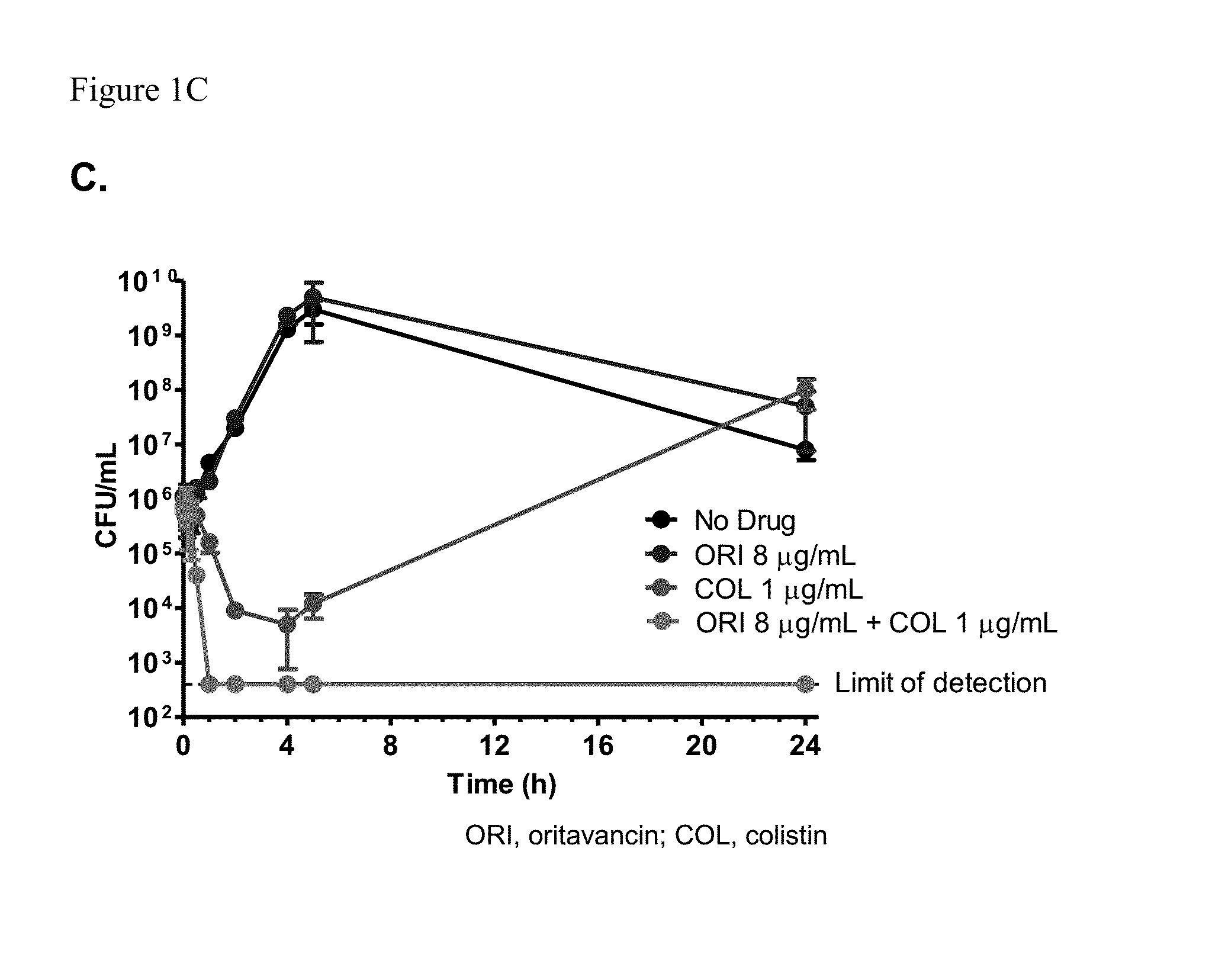
Methods for treating bacterial infections using oritavancin and polymyxins
Methods of treating bacterial infections in a subject using a synergistic combination of oritavancin and a polymyxin are disclosed.
Owner:MELINTA THERAPEUTICS
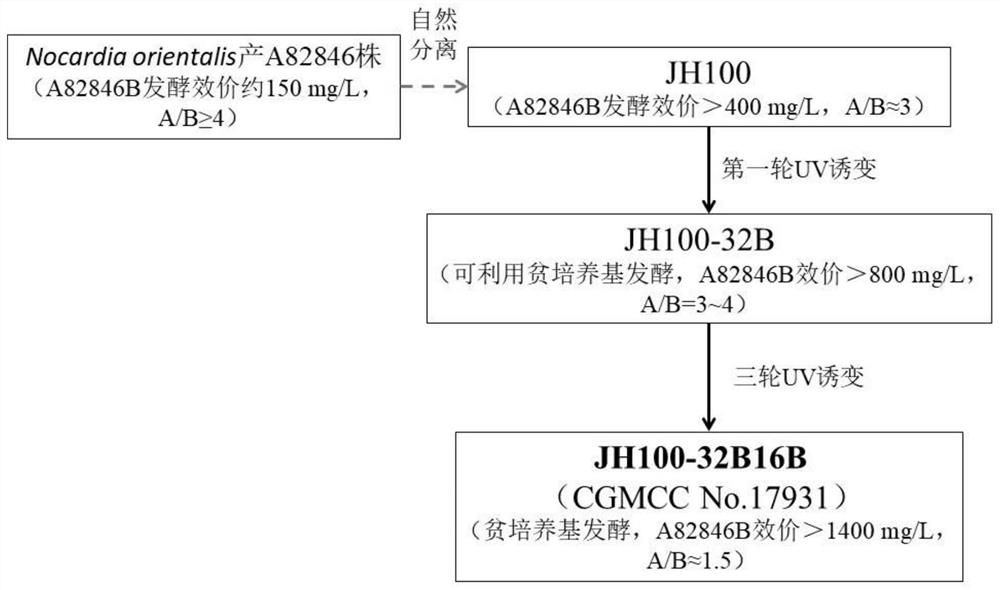
Producing strain of oritavancin intermediate and application of producing strain
PendingCN113493748AStable genetic traitsLow costBacteriaMicroorganism based processesBiotechnologyMicroorganism
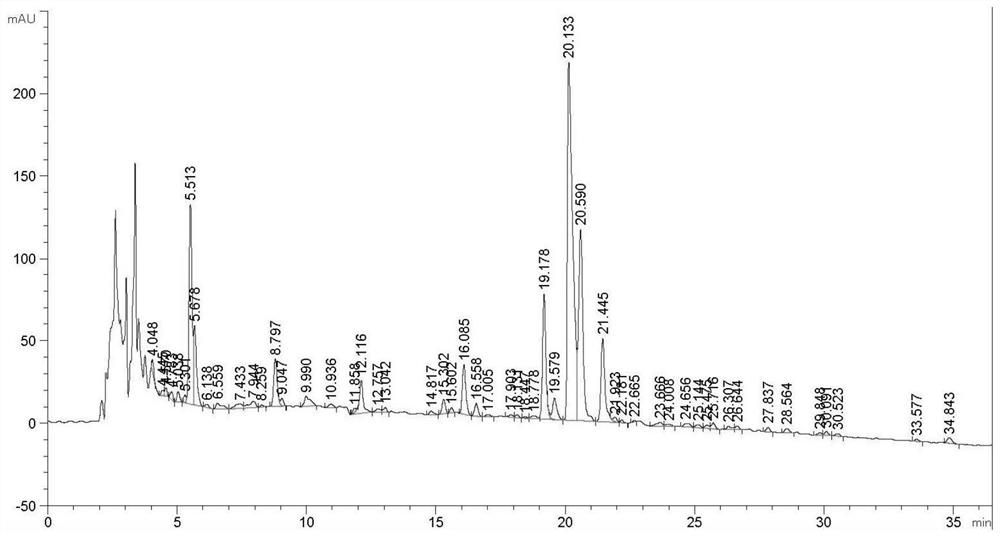
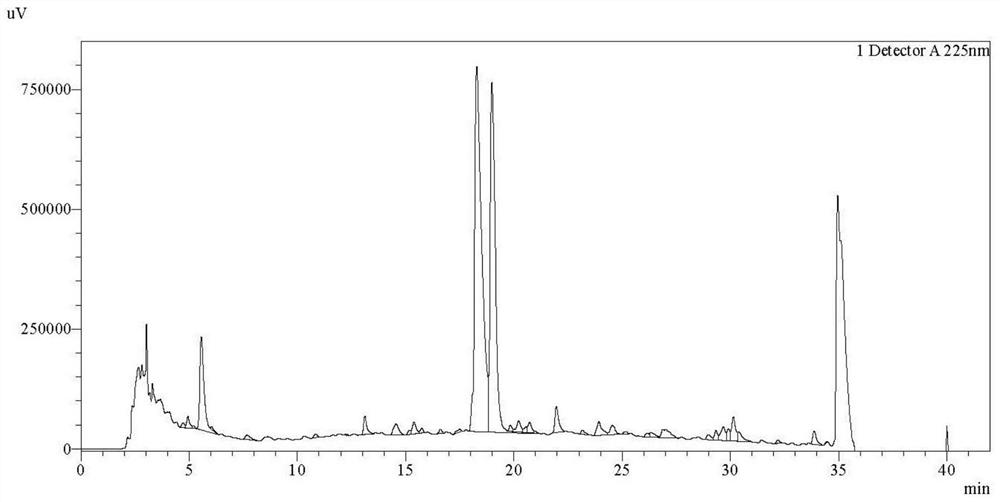
The invention discloses a producing strain of an oritavancin intermediate (A82846B) and application of the producing strain. The producing strain kibdelosporangium aridum JH100-32B16B of the oritavancin intermediate is preserved in China General Microbiological Culture Collection Center (CGMCC), and the preservation number is CGMCC No.17931. The fermentation method is a method for producing the oritavancin intermediate by fermenting the kibdelosporangium aridum CGMCC No.17931 in a fermentation culture medium. By adopting the producing strain and a corresponding fermentation process thereof, the fermentation titer of A82846B can be 1450mg / L on a 30L fermentation tank by adopting a fermentation culture medium with a low-cost formula, and the fermentation cost is reduced by more than 90% compared with that of a contrast process; and the "impurity-product ratio" (A / B ratio) is as low as 1.5-2.0, and is reduced by more than half compared with a control level, thereby being beneficial to simplifying a product extraction process and reducing the extraction cost. The advantages in two aspects are combined, so that the comprehensive production cost of the A82846B is obviously lower than a control process level reported in the existing literature.
Owner:SHANGHAI JIANHE PHARM & TECH CO LTD
Method of inhibiting clostridium difficile by administration of oritavancin
Glycopeptide antibiotics, such as oritavancin, demonstrate significant activity against both a vegetative form of C. difficile and C. difficile spores. Methods for the treatment, prophylaxis and prevention of C. difficile infection and disease in animals, including humans, are described.
Owner:MELINTA THERAPEUTICS
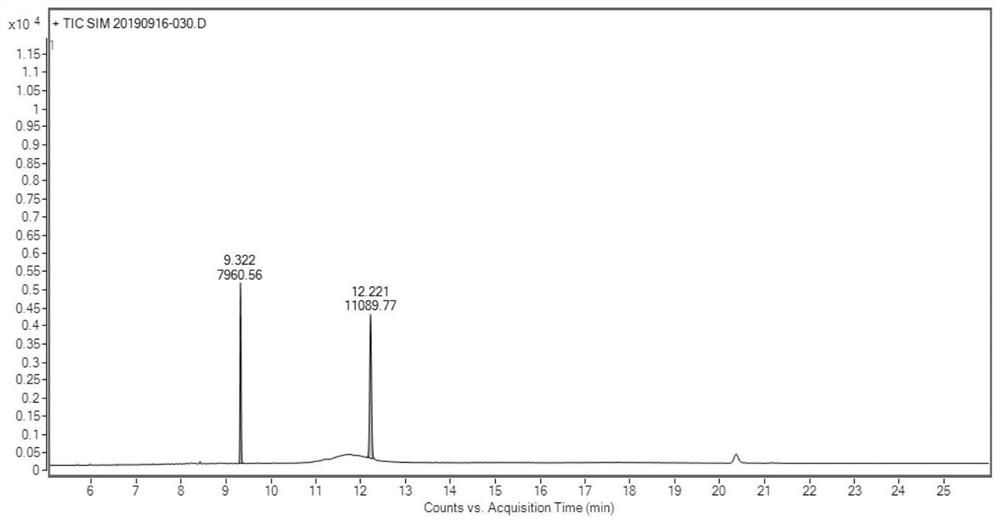
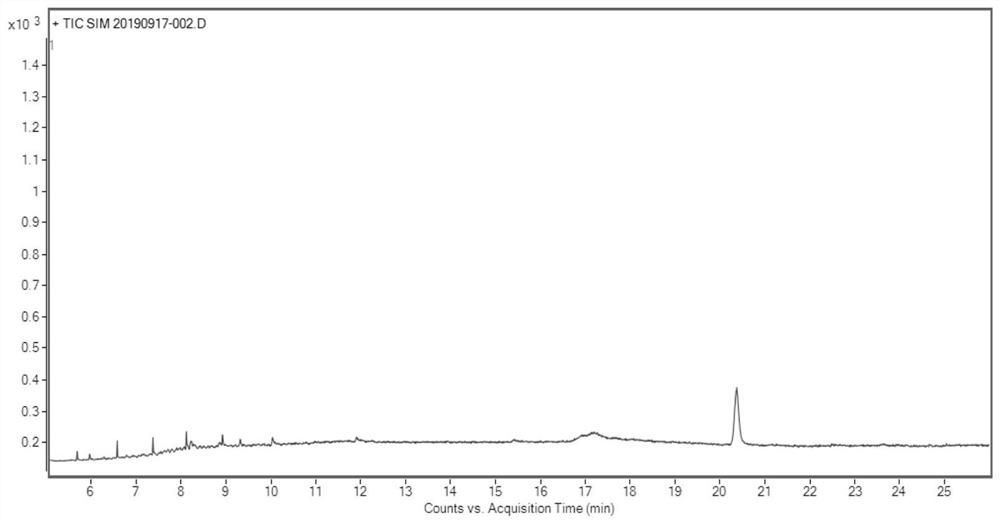
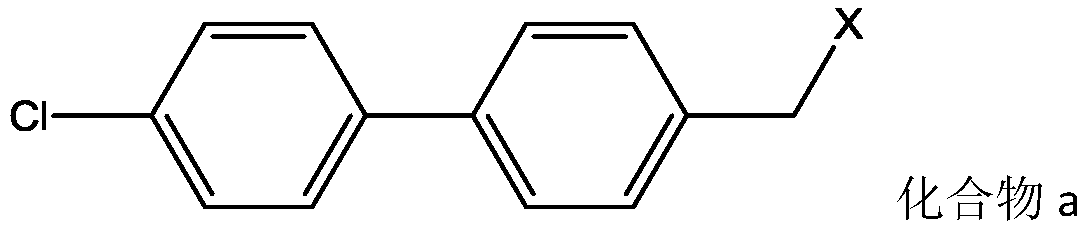
Determination method of oritavancin intermediate impurity 4-chlorobiphenyl and 4,4-dichlorobiphenyl content
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST
Methods for treating bacterial infections using oritavancin and polymyxins
Methods of treating bacterial infections in a subject using a synergistic combination of oritavancin and a polymyxin are disclosed.
Owner:MELINTA THERAPEUTICS
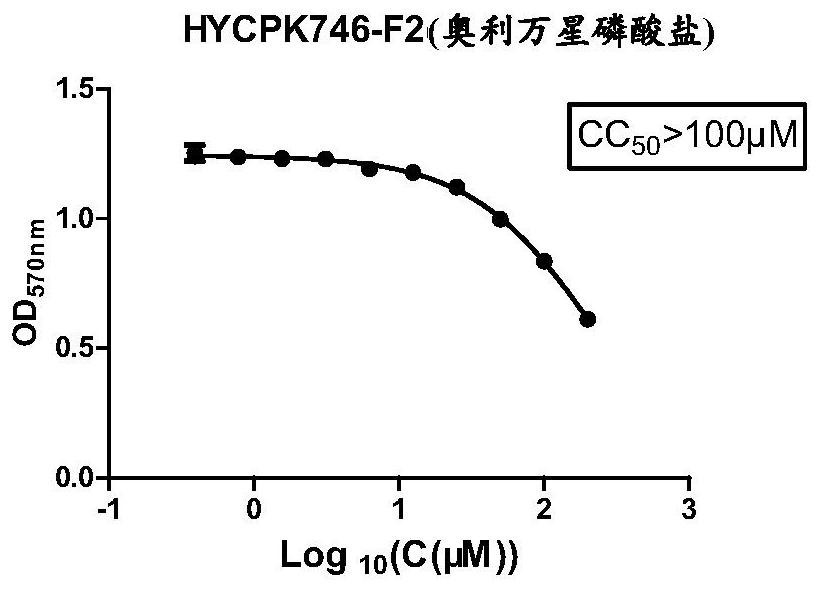
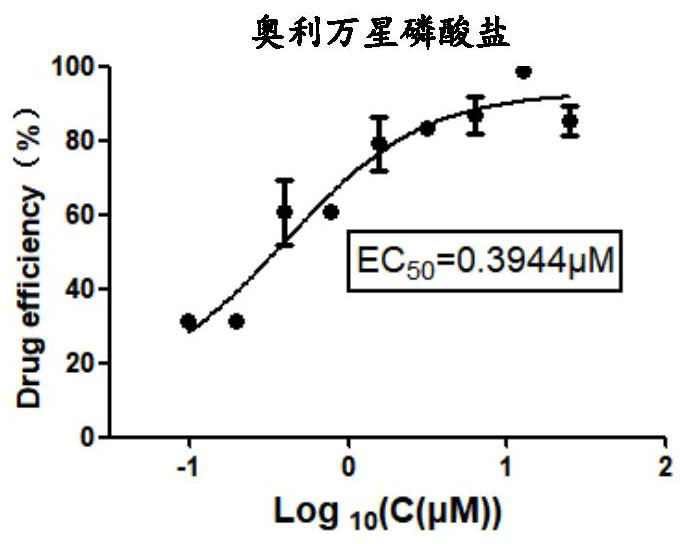
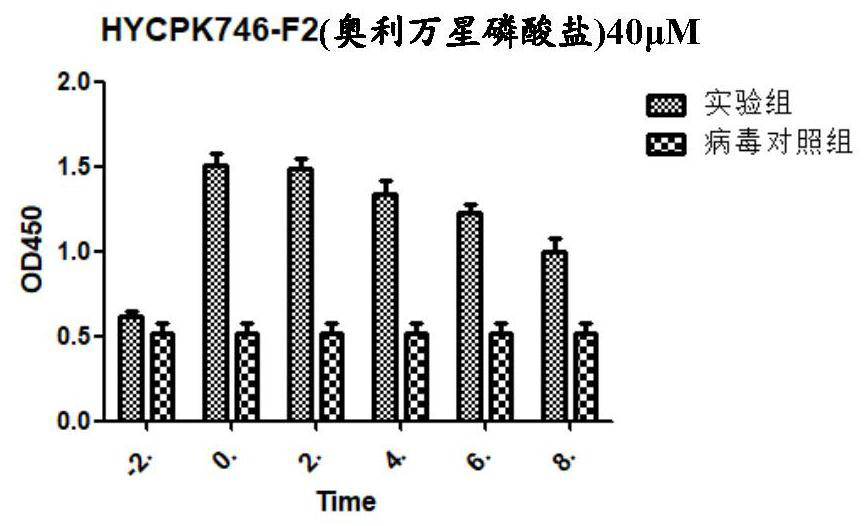
Application of Oritavancin Phosphate in Preparation of Drugs for Prevention and Treatment of Bovine Infectious Rhinotracheitis
ActiveCN109550041BAntiviralsSaccharide peptide ingredientsOritavancinInfectious bovine rhinotracheitis virus
The invention provides an application of oritavancin phosphate in the preparation of a drug for preventing and treating bovine infectious rhinotracheitis. Oritavancin phosphate can inhibit and kill bovine infectious rhinotracheitis in an in vitro MDBK cell model The virus can effectively inhibit the invasion and replication of bovine infectious rhinotracheitis virus, and has low cytotoxicity. It also lays the foundation for the development of highly effective and specific anti-bovine infectious rhinotracheitis virus drugs and provides a new perspective.
Owner:DAIRY CATTLE RES CENT SHANDONG ACADEMY OF AGRI SCI +1
Method for preparing oritavancin by means of carboxyl protecting
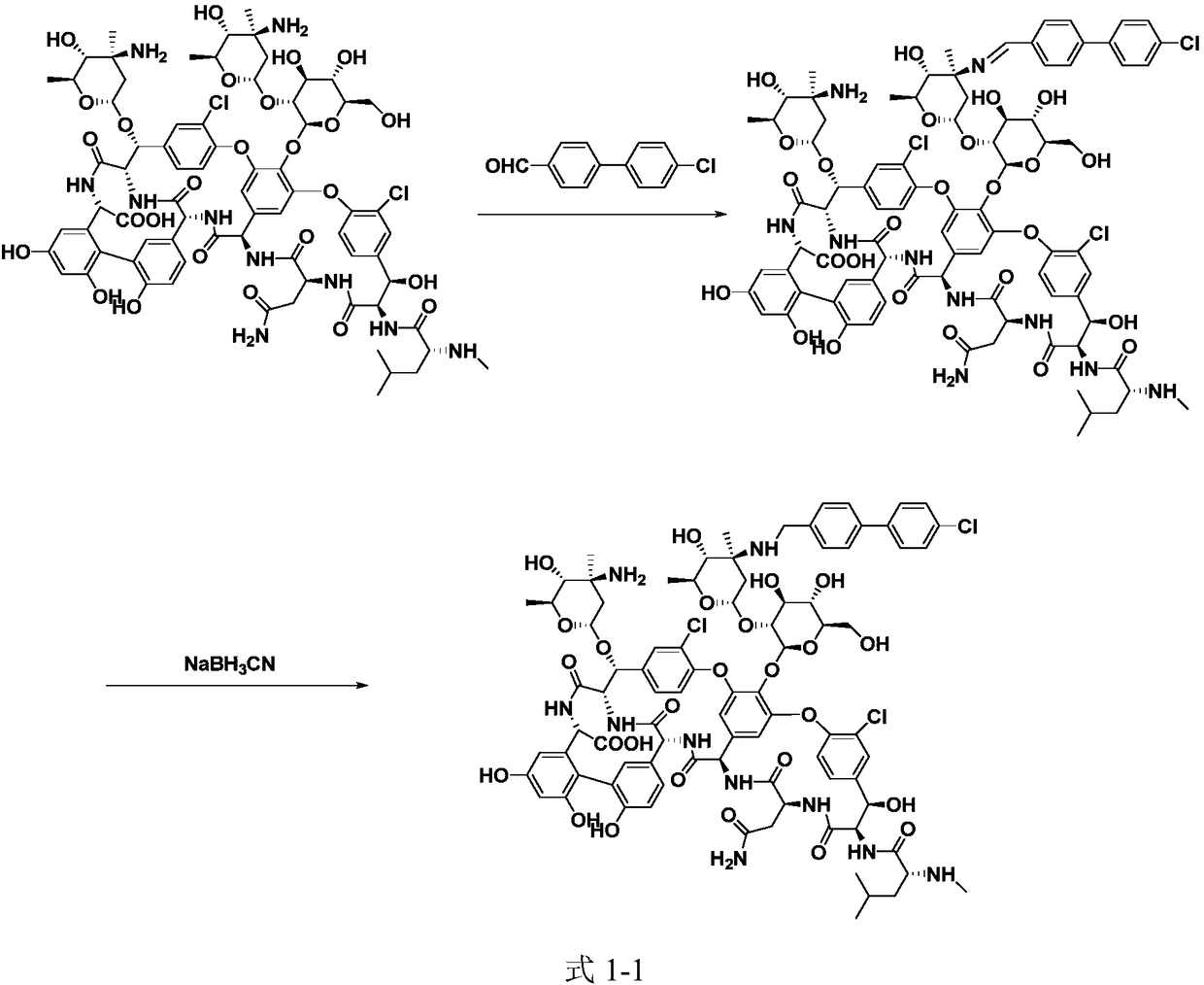
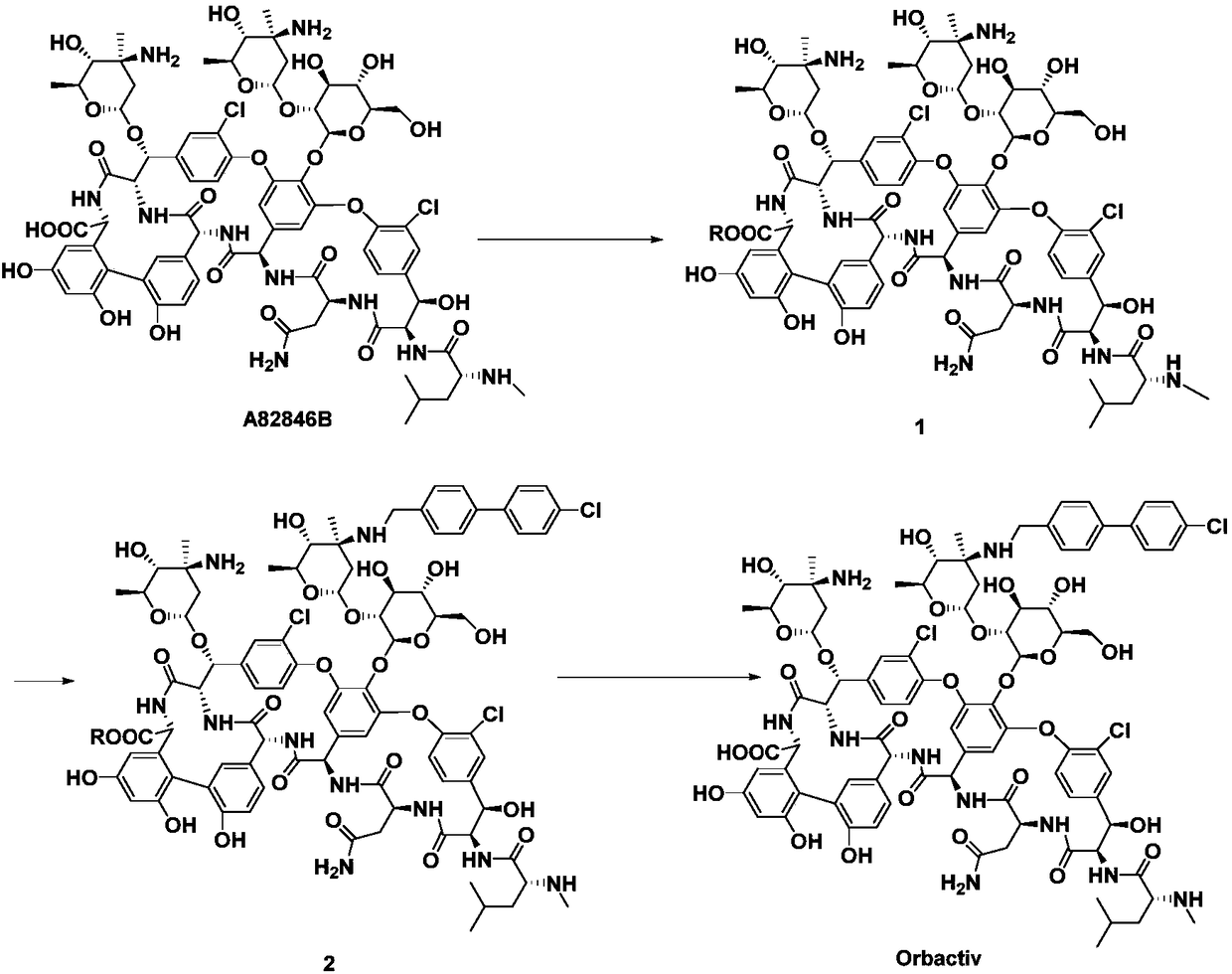
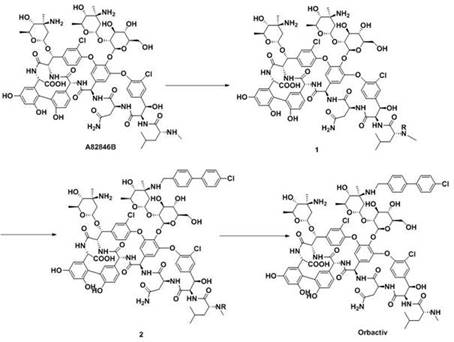
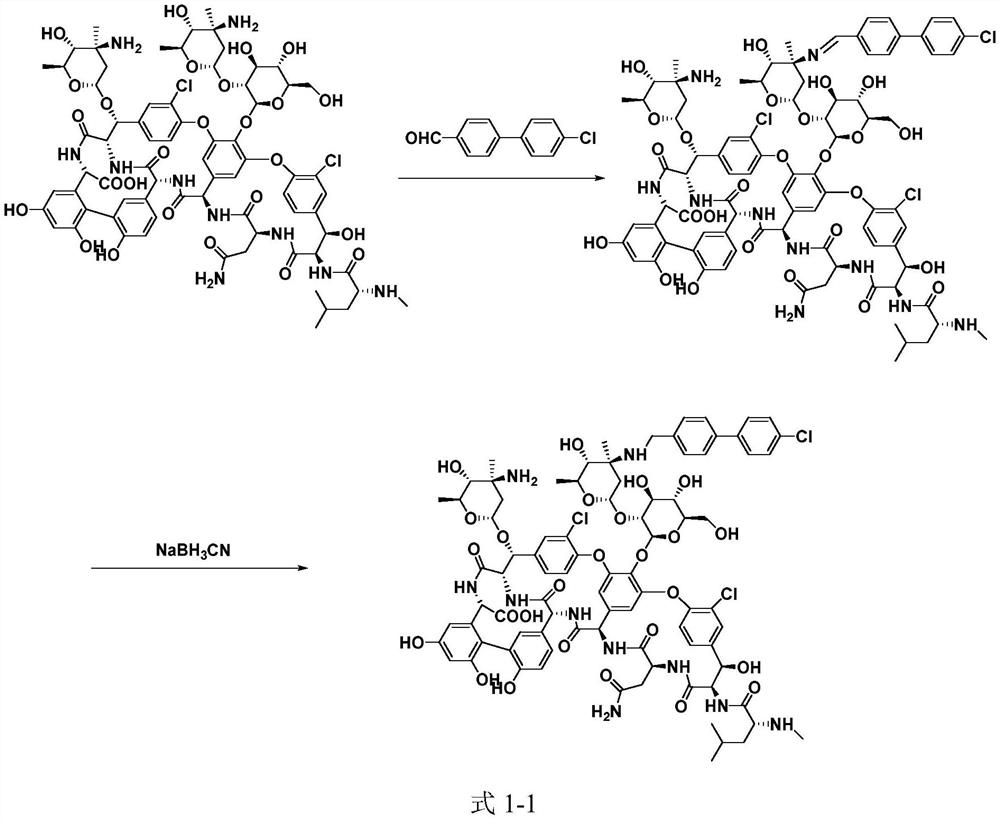
ActiveCN109053865ANo reduction in final yieldHigh yieldPeptide preparation methodsBulk chemical productionOritavancinProtecting group
The invention discloses a method for preparing oritavancin by means of carboxyl protecting. The method comprises the steps that carboxyl of A82846B is protected, then the A82846B and 4'-chlorobiphenyl-4-carbaldehyde are subjected to aldehyde-amine condensation, then a C=N bond is reduced, and finally, a carboxyl protecting group is removed to obtain the oritavancin. By means of the method for preparing the oritavancin by means of carboxyl protecting, limitation of the prior art is broken through, in an oritavancin synthesis process, operation of protecting the carboxyl of the A82846B and subsequent synthesis operation of conducting aldehyde-amine condensation on the A82846B and the 4'-chlorobiphenyl-4-carbaldehyde and the like are added, accidentally, the reaction selectivity is improved,not only is the final yield of the oritavancin not reduced, but also the efficiency of subsequent reaction is effectively improved, by-products are reduced, and the yield and the purity of the oritavancin are improved, so that the method has the unexpected effects.
Owner:LIVZON NEW NORTH RIVER PHARMA
Method for preparing oritavancin intermediate by fermentation
ActiveCN109811024BIncrease the proportion of componentsHigh yieldMicroorganism based processesPeptidesBiotechnologyOritavancin
Owner:JIANGSU SENRAN CHEM
A kind of method for preparing oritavancin
ActiveCN109053864BHigh yieldHigh purityPeptide preparation methodsBulk chemical productionBiochemical engineeringOritavancin
The invention discloses a method for preparing oritavancin. The present invention breaks through the limitations of the prior art. In the synthesis process of oritavancin, the amine group at a specific site is first protected, and the synthesis operation steps are increased, and then subsequent synthesis operations are performed, which not only does not reduce the final product efficiency, while effectively improving the efficiency of subsequent reactions, reducing the generation of by-products, but improving the yield and purity of oritavancin.
Owner:LIVZON NEW NORTH RIVER PHARMA
A pharmaceutical composition capable of resisting chronic infection and biofilm bacteria and its application
ActiveCN110201174BRelieve inflammationAntibacterial agentsOrganic active ingredientsOritavancinMeropenem
The invention provides a pharmaceutical composition capable of resisting chronic infection and biofilm bacteria, the active ingredients of which include clinfloxacin and at least one antibiotic selected from daptomycin and oritavancin; The active ingredient of the composition also includes an additional antibiotic, and the additional antibiotic is at least one antibiotic selected from vancomycin and meropenem. By using the pharmaceutical composition of the present invention, persistent bacteria can be completely eliminated in vitro, and persistent biofilm infection can be effectively eliminated, the lesion can be cured, and inflammation can be relieved; in addition, the pharmaceutical composition of the present invention is suitable for infections in vivo, in vitro, and on the body surface Treatment has a wide range of applications.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
A preparation method of high-purity oritavancin key intermediate a82846b
The invention provides a method for preparing the high-purity oritavancin key intermediate A82846B, specifically by separating the oritavancin key intermediate A82846B from the fermentation broth, first adjusting the pH, and then purifying the oritavancin key intermediate through a macroporous adsorption resin , then purified by reverse phase chromatography, and finally crystallized to obtain high-purity A82846B. The method adopted in the present invention is simple to operate, uses less organic solvents, and greatly reduces the generation of waste liquid; and overcomes the defects of low adsorption efficiency of cationic macroporous adsorption resins in the prior art and reduced product yield due to leakage adsorption, and is suitable for Industrial production.
Owner:CHONGQING QIANTAI BIOLOGICAL MEDICINE
Use of oritavancin for prevention and treatment of anthrax
Glycopeptide antibiotics, such as oritavancin, demonstrate significant activity against B. anthracis. Methods for the treatment, prophylaxis and prevention of B. anthracis infection and disease in animals, including humans, are described.
Owner:MELINTA THERAPEUTICS
an alkaline degradation solution
ActiveCN109762860BIncrease the proportion of componentsHigh yieldMicroorganism based processesPeptidesOritavancinPhysical chemistry
The invention discloses an alkaline degradation liquid for preparing oritavancin intermediate A82846B by fermentation. The impurity waste liquid is subjected to alkali degradation to obtain an alkali degradation liquid. After the alkali degradation liquid was added to the fermentation medium, the fermentation was carried out again. By adding different concentrations of impurity alkali degradation liquid, the fermentation unit of A82846B was greatly increased, and the components were significantly improved.
Owner:JIANGSU SENRAN CHEM
A kind of preparation method of oritavancin
The invention discloses an improved method for preparing oritavancin. The method takes A82846B acetate as a raw material, under existence of a Brettphos catalyst, a Brettphos ligand, alkali and a solvent, the material and an amino alkylation reagent are subjected to a reaction to generate oritavancin with one step. According to the invention, reaction steps are simplified, the selectivity of amino alkylation is increased, oritavancin yield is increased, and a route suitable for large industrial production is provided.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
A kind of preparation method of desert pseudocystoid and oritavancin intermediate
ActiveCN106190854BEase of industrial productionIncrease productionFungiMicroorganism based processesMicroorganismOritavancin
The invention discloses kibdelosporangium aridum and a preparation method of an oritavancin intermediate. The kibdelosporangium aridum strain HS807-AN-2396 with high yield of the oritavancin intermediate is preserved in the China General Microbiological Culture Collection Center (CGMCC), and is assigned with the accession number of CGMCC No.10576. The preparation method of the oritavancin intermediate comprises the following step: fermenting the kibdelosporangium aridum CGMCC No.10576 in a fermentation medium, thus obtaining the oritavancin intermediate A82846B from the fermentation broth. With the adoption of the preparation method, the high yield, namely, 2315mg / L of the fermentation potency of the oritavancin intermediate can be realized on a 60m<3> tank, and thus the industrial production of oritavancin is facilitated.
Owner:ZHEJIANG HISUN PHARMA CO LTD
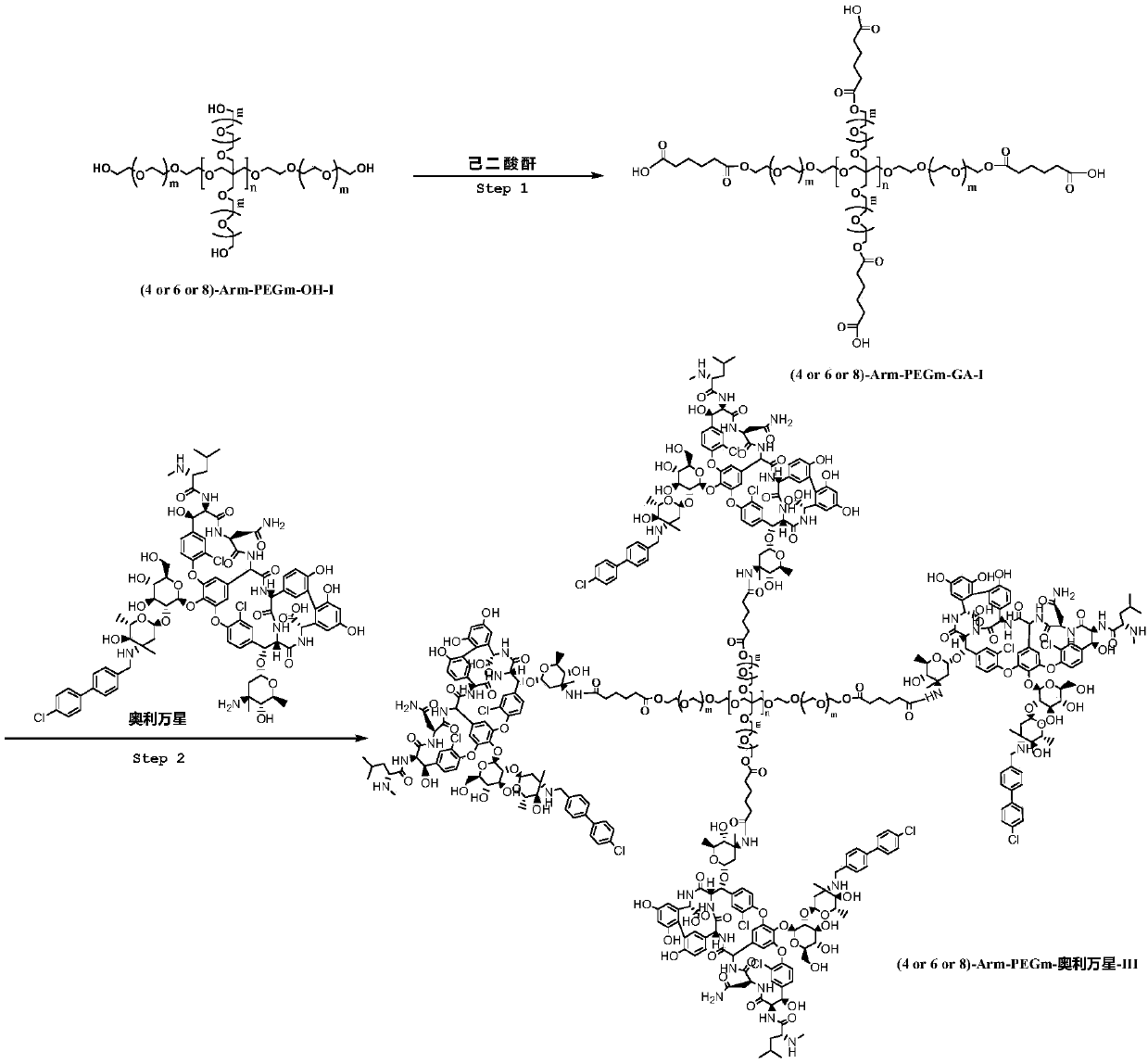
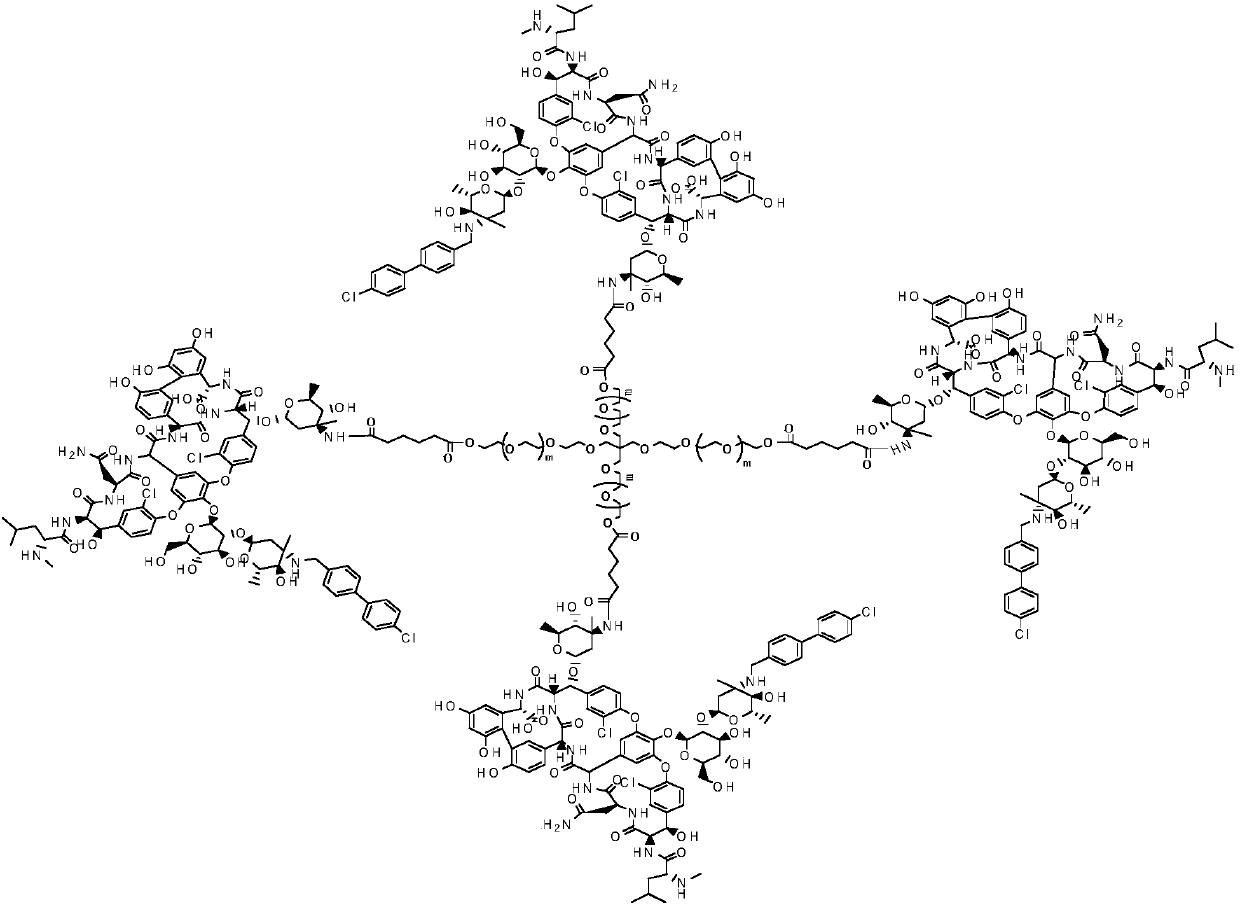
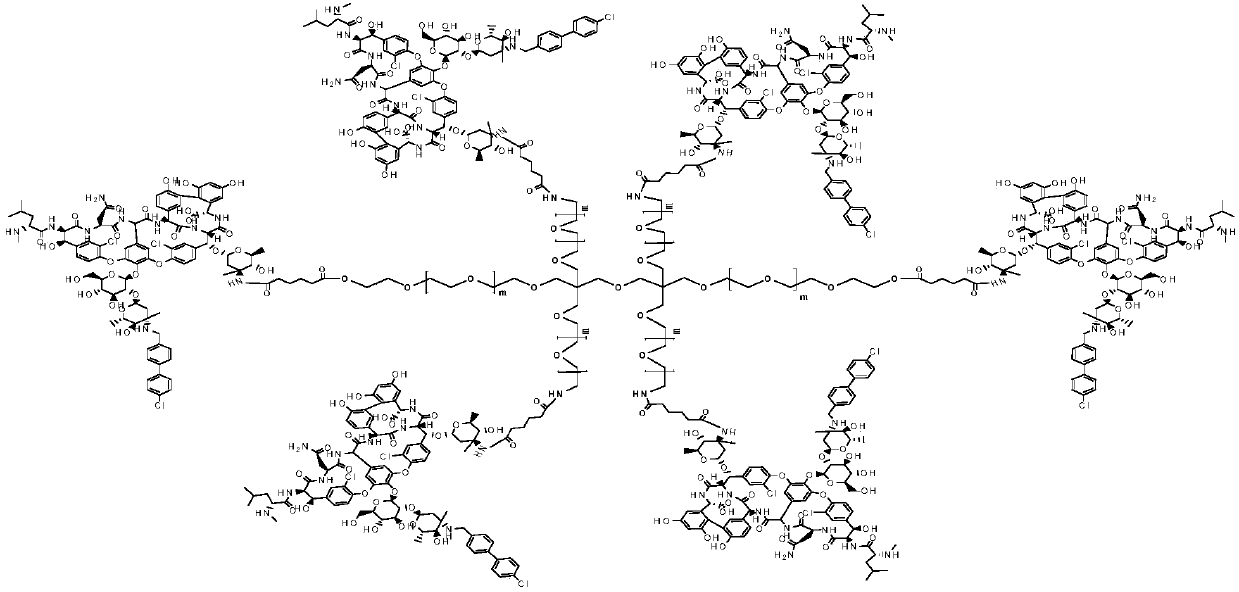
Multi-arm PEGylated oritavancin derivative and preparation method thereof
ActiveCN110643034AGood water solubilityHigh load rateAntibacterial agentsAntisepticsOritavancinPolyethylene glycol
According to the invention, four-arm PEG (polyethylene glycol), six-arm PEG and multi-arm PEG are respectively connected with oritavancin by utilizing the characteristics that the multi-arm PEG is non-toxic and easy to combine. A multi-arm PEG loaded oritavancin prodrug not only has good water solubility, but also is most importantly characterized in that one multi-arm PEG chain can be connected with a plurality of oritavancin residues, so that the loading rate of the drug is greatly increased, the half-life period of the drug is greatly prolonged, the existence time of the drug in plasma is remarkably prolonged, and the curative effect is improved.
Owner:湖南华腾医药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com