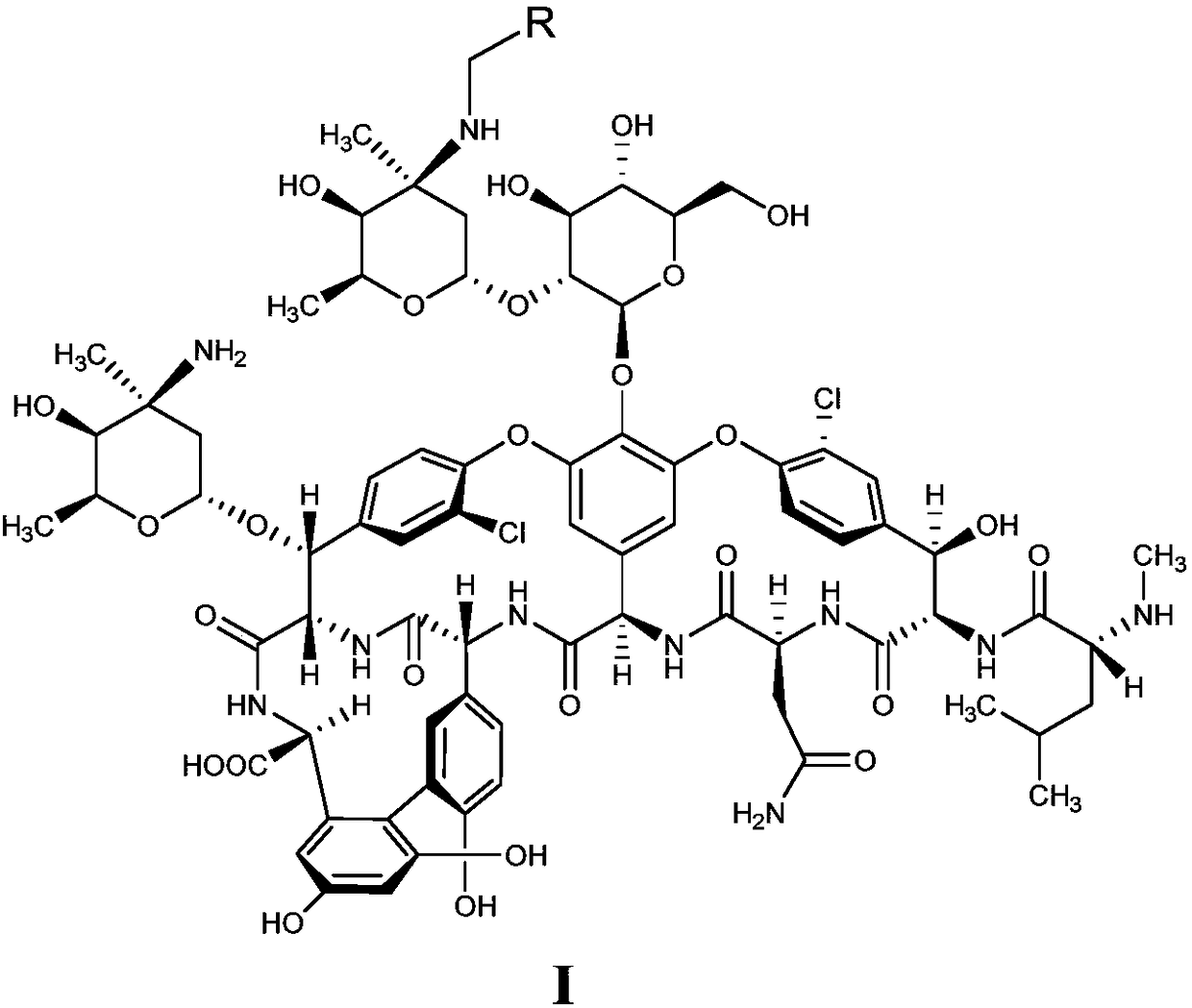
Glycopeptides compounds with anti-resistance bacterial activity, preparation method and application thereof
A technology of anti-drug-resistant bacteria and compounds, which is applied in the field of drugs for the treatment of infectious diseases and novel glycopeptide compounds, can solve the problems of reduced activity and achieve high safety and high inhibitory activity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1, the preparation of compound 1
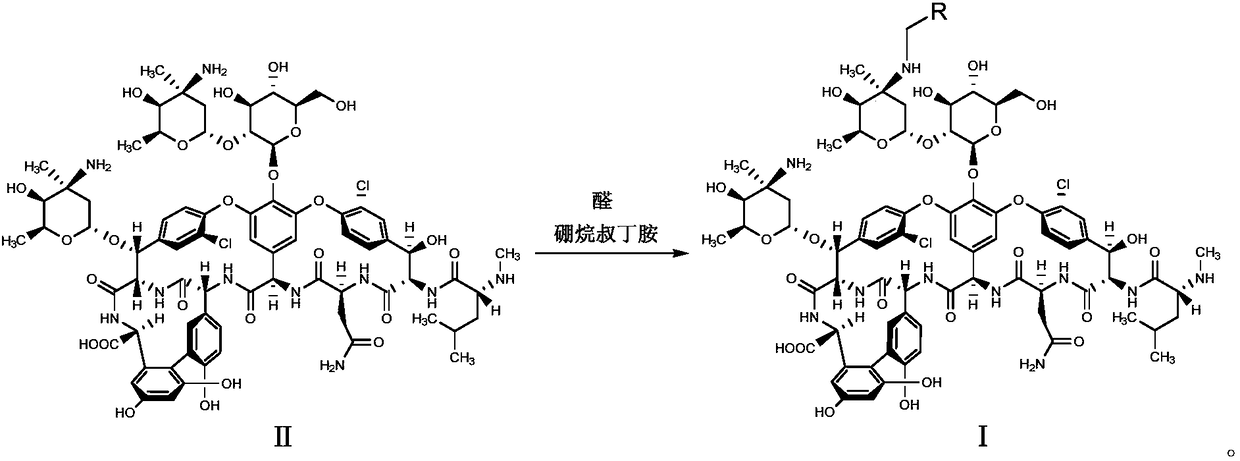
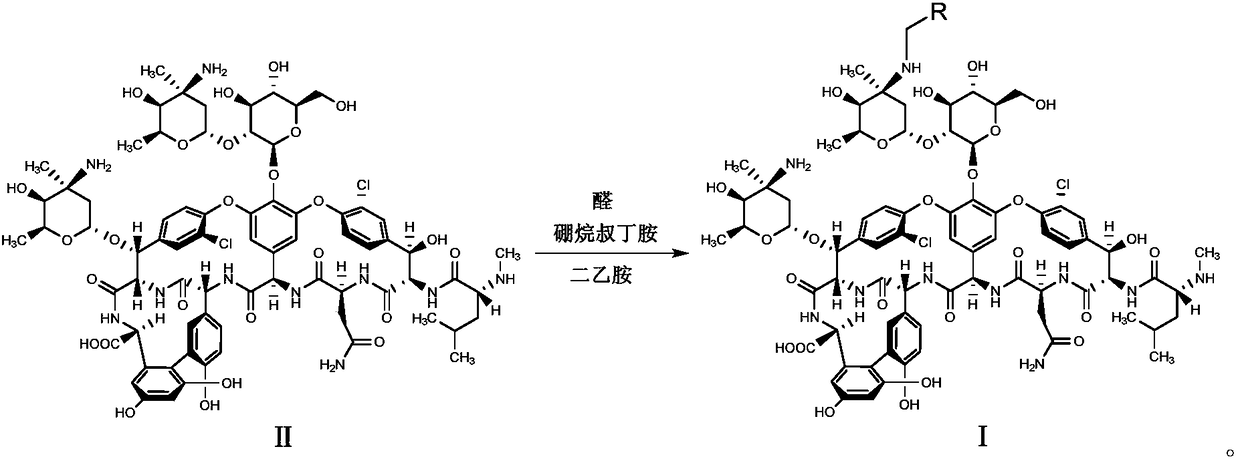
[0043] Mix compound II (0.5g, 0.3mmol) with 10mL DMF-methanol (1:1, v / v), add DIEA (0.1mL, 0.6mmol) and 4-benzyloxybenzaldehyde (0.085g, 0.4mmol ), stirred at 65°C for 2h and cooled to room temperature, added TFA (0.07mL, 0.9mmol) and borane tert-butylamine (0.05g, 0.6mmol) and continued to stir at room temperature for 2h, and added methyl tert-butyl ether to the reaction solution (50mL), the precipitate was collected by suction filtration, the residue was purified with a reverse-phase polymer filler, eluted with methanol-0.04% TFA aqueous solution (1:4, v / v), concentrated and dried to obtain compound 1 (white solid 0.28g, Yield 52%).
[0044] C 87 h 100 Cl 2 N 10 o 27 Molecular weight calculated value: 1786.61, measured value: m / z=1787.60 [M+H] + .
Embodiment 2
[0045] Embodiment two, the preparation of compound 2
[0046] Mix compound II (0.5g, 0.3mmol) with 10mL DMF-methanol (1:1, v / v), add DIEA (0.1mL, 0.6mmol) and 4-phenylethoxybenzaldehyde (0.09g, 0.4 mmol), stirred at 65°C for 2h and cooled to room temperature, added TFA (0.07mL, 0.9mmol) and borane tert-butylamine (0.05g, 0.6mmol) and continued to stir at room temperature for 2h, and added methyl tert-butyl to the reaction solution Ether (50mL), the precipitate was collected by suction filtration, the residue was purified by reverse phase polymer packing, eluted with methanol-0.04% TFA aqueous solution (1:4, v / v), concentrated and dried to obtain compound 2 (white solid 0.31g , yield 57%).
[0047] C 88 h 102 Cl 2 N 10 o 27 Molecular weight calculated value: 1800.63, measured value: m / z=1801.63 [M+H] + .
Embodiment 3
[0048] Embodiment three, the preparation of compound 3
[0049] Mix compound II (1.0g, 0.6mmol) with 15mL DMF-methanol (1:1, v / v), add DIEA (0.2mL, 1.2mmol) and 4-phenylpropoxybenzaldehyde (0.2g, 0.8 mmol), stirred at 65°C for 2h, cooled to room temperature, added TFA (0.14mL, 1.8mmol) and borane tert-butylamine (0.1g, 1.2mmol) and continued to stir at room temperature for 2h, then added methyl tert-butyl ether (70mL ), the precipitate was collected by suction filtration, the residue was purified by reverse phase polymer packing, eluted with methanol-0.04% TFA aqueous solution (1:4, v / v), concentrated and dried to obtain compound 3 (white solid 0.65g, yield 60%).
[0050] C 89 h 104 Cl 2 N 10 o 27 Molecular weight calculated value: 1814.64, measured value: m / z=1815.64 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com