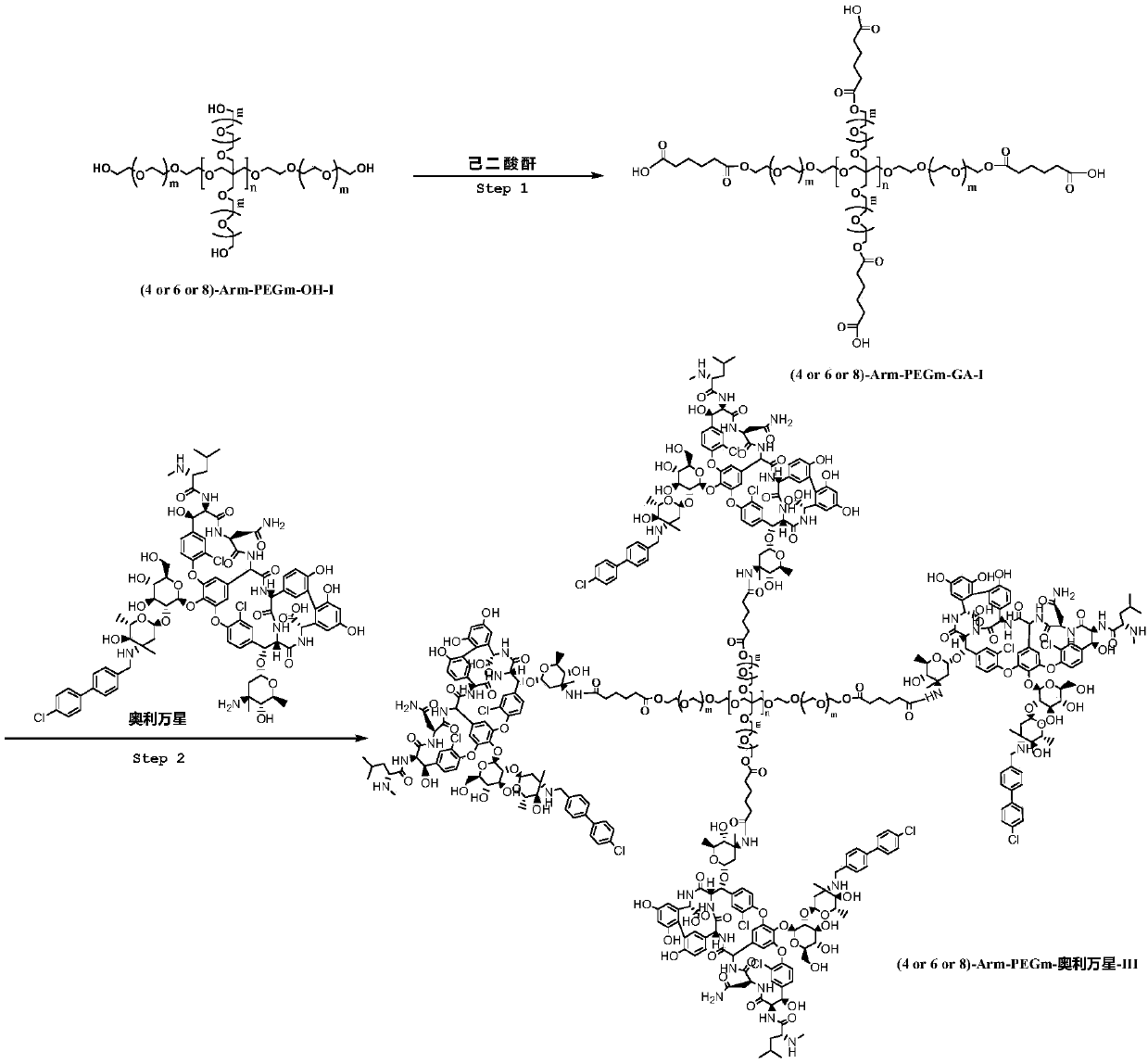
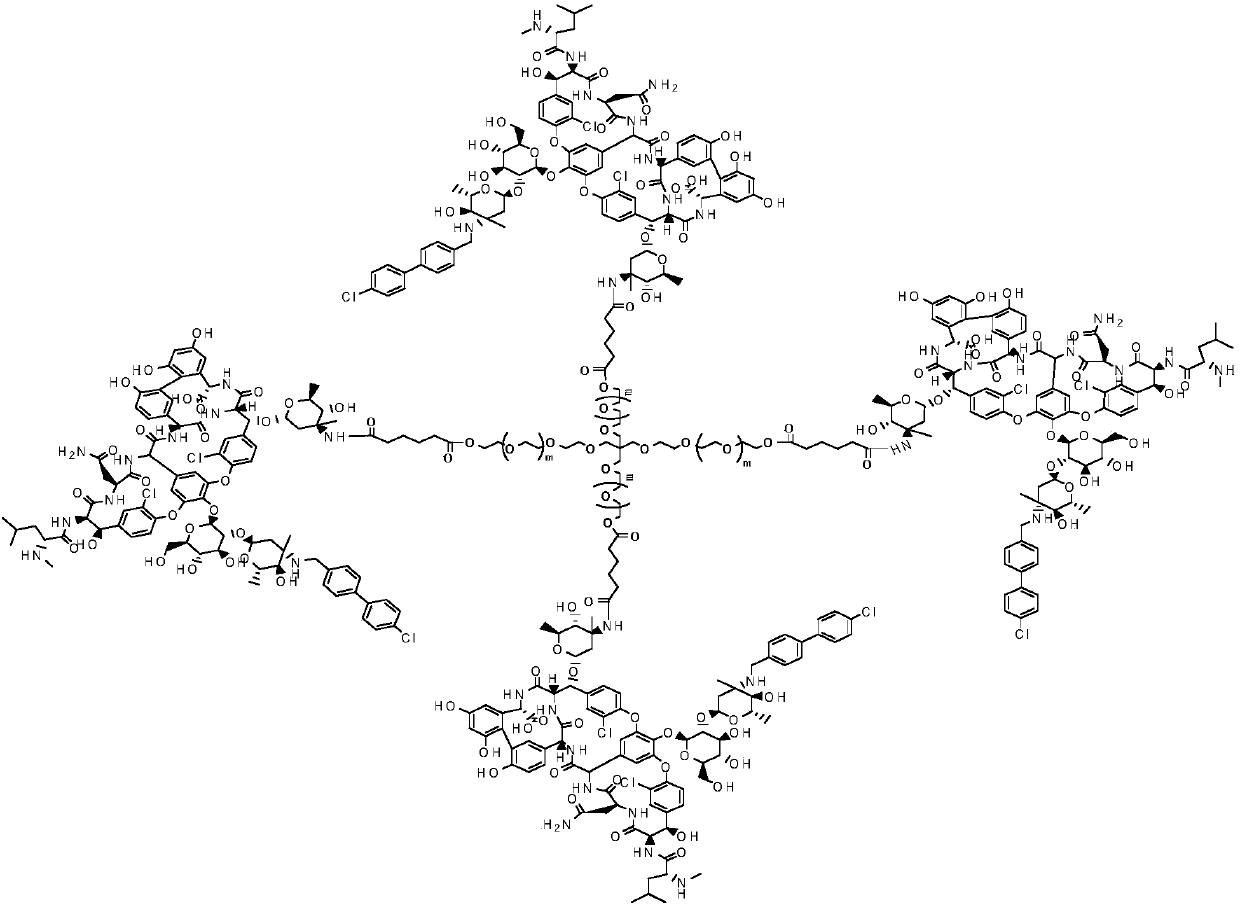
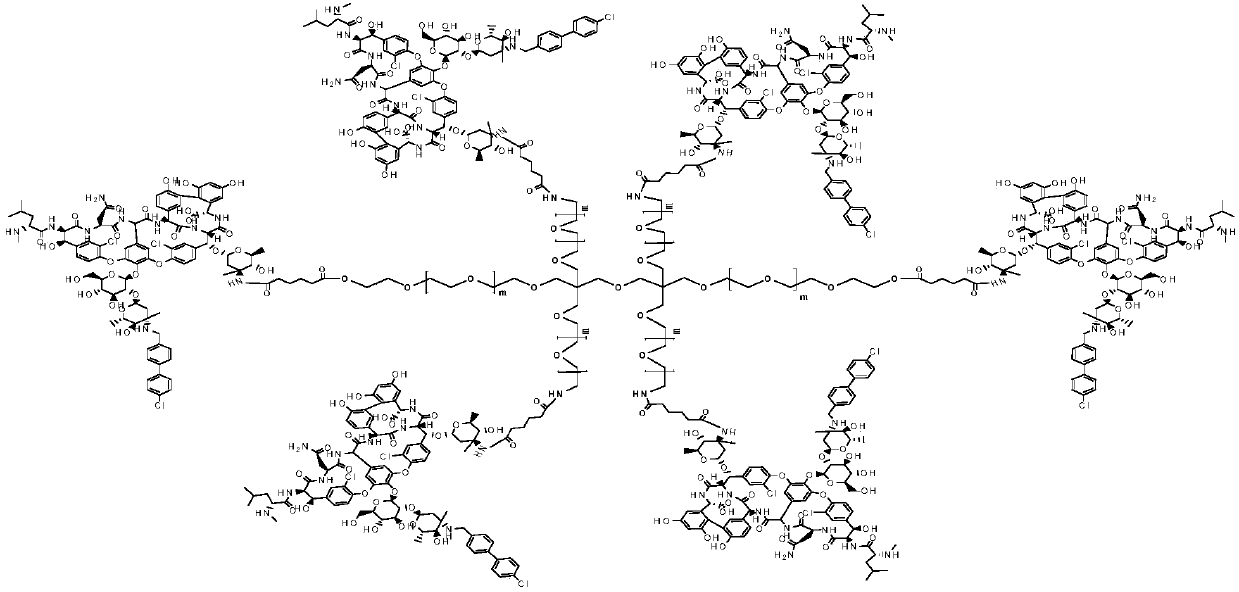
Multi-arm PEGylated oritavancin derivative and preparation method thereof
A technology of oritavancin and derivatives, applied in the preparation and treatment of acute bacterial skin and skin structure infections, multi-arm PEGylated oritavancin derivatives and their preparation and preparation fields, can solve the problem of loading limited quantity and other problems, to achieve the effect of easy quality control, good in vitro and in vivo activity of the drug, and reduced cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Preparation of 4-Arm-PEG24-GA-II
[0029] Dissolve 10mmol of 4-Arm-PEG24-OH-I in 100ml of DMF, add 11mmol of adipic anhydride and 10mmol of DMAP at room temperature, and then stir at 100°C for 10h. After the reaction was completed, the solution was distilled off under reduced pressure to obtain a crude product. The crude product was purified by chromatography to obtain 4-Arm-PEG24-GA-II. Yield: 90%. NMR data are as follows: 1H NMR (400MHz, CDCl3) δ4.26(s,2H),3.66(m,96H),2.86(s,2H),2.74(s,2H),2.52(s,2H), 2.09( s,2H).
[0030] (2) Preparation of 4Arm-PEG24-oritavancin-II
[0031] Dissolve 10mmol of 4-Arm-PEGm-GA-II in 50ml of N,N-dimethylformamide, add 10mmol of DIC and 10mmol of 4-PPY, and stir at 25°C for 2h. 80 mmol of oritavancin was added to the reaction, and the reaction was carried out at 30°C for 12 hours. After the completion of the oritavancin reaction as detected by TLC tracking, 4Arm-PEG24-oritavancin-II was obtained by recrystallization and chromato...
Embodiment 2
[0033] (1) Preparation of 4-Arm-PEG124-GA-II
[0034] Dissolve 10mmol of 4-Arm-PEG124-OH-I in 100ml of DMF, add 11mmol of adipic anhydride and 10mmol of DMAP at room temperature, and then stir at 100°C for 8h. After the reaction was completed, the solution was distilled off under reduced pressure to obtain a crude product. The crude product was purified by chromatography to obtain (4or 6or 8)-Arm-PEGm-GA-II. Yield: 90%. NMR data are as follows: 1H NMR (400MHz, CDCl3) δ4.26(s,2H),3.66(m,496H),2.86(s,2H),2.74(s,2H),2.52(s,2H),2.09( s,2H).
[0035] (2) Preparation of 4Arm-PEG124-oritavancin-II
[0036] Dissolve 10mmol of 4-Arm-PEG24-GA-II in 50ml of N,N-dimethylformamide, add 10mmol of DIC and 10mmol of 4-PPY, and stir at 25°C for 2h. 80 mmol of oritavancin was added to the reaction, and the reaction was carried out at 30°C for 12 hours. After TLC tracking detection oritavancin reaction was complete, 4Arm-PEG124-oritavancin-II was obtained by recrystallization and chromatog...
Embodiment 3
[0038] (1) Preparation of 4-Arm-PEG240-GA-II
[0039] Dissolve 10mmol of 4-Arm-PEG240-OH-I in 100ml of DMF, add 11mmol of adipic anhydride and 10mmol of DMAP at room temperature, and then stir at 120°C for 10h. After the reaction was completed, the solution was distilled off under reduced pressure to obtain a crude product. The crude product was purified by chromatography to obtain 4-Arm-PEG240-GA-II. Yield: 90%. NMR data are as follows: 1H NMR (400MHz, CDCl3) δ4.26(s, 2H), 3.66(m, 960H), 2.86(s, 2H), 2.74(s, 2H), 2.52(s, 2H), 2.09( s,2H).
[0040] (2) Preparation of 4Arm-PEG240-oritavancin-II
[0041] Dissolve 10mmol of 4-Arm-PEG240-GA-II in 50ml of N,N-dimethylformamide, add 10mmol of DIC and 10mmol of 4-PPY, and stir at 25°C for 2h. 80 mmol of oritavancin was added to the reaction, and the reaction was carried out at 30°C for 12 hours. After TLC tracking detection oritavancin reaction was complete, 4Arm-PEG240-oritavancin-II was obtained by recrystallization and chromat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com