Kibdelosporangium aridum and preparation method of oritavancin intermediate
A technology of oritavancin and cystobacteria, applied in the field of medicine, can solve the problems of small-scale excision, frameshift mutation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
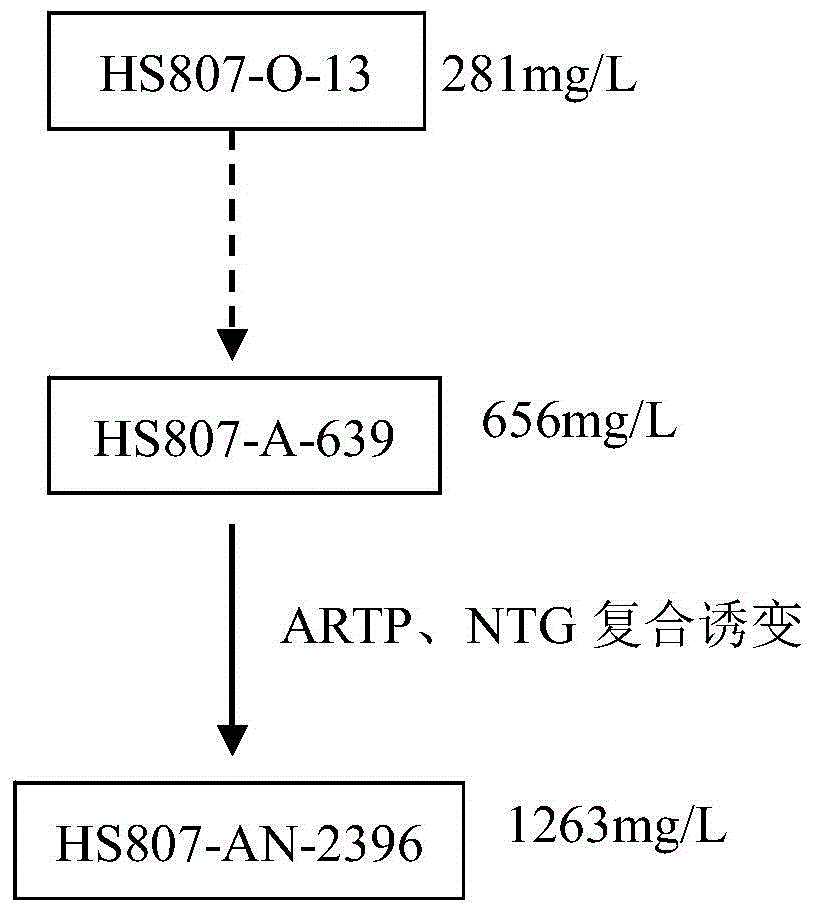
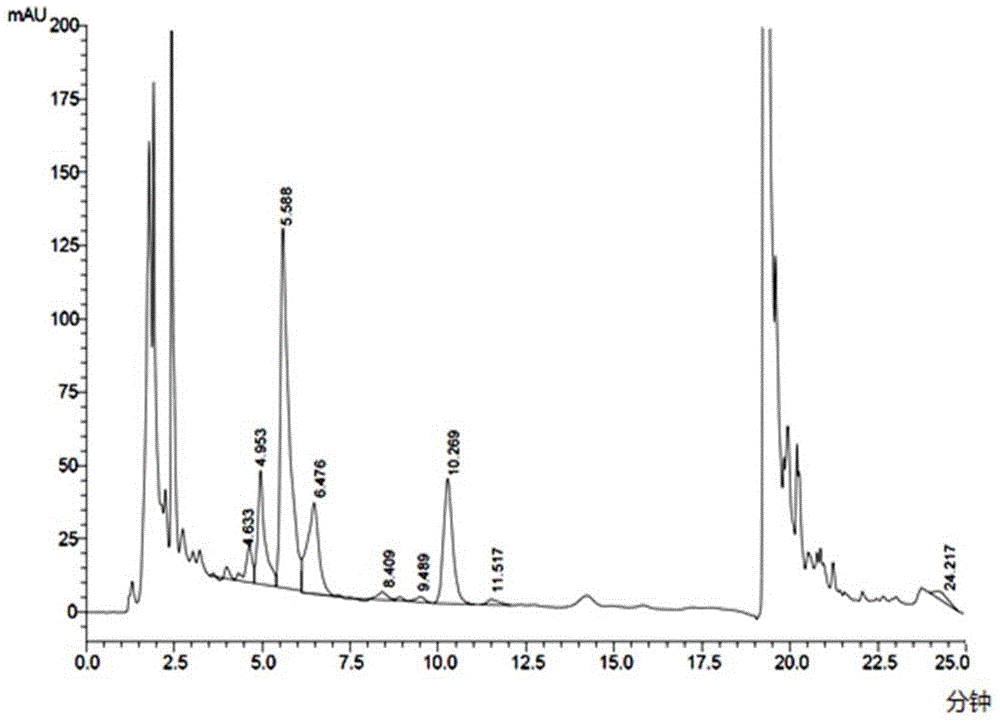
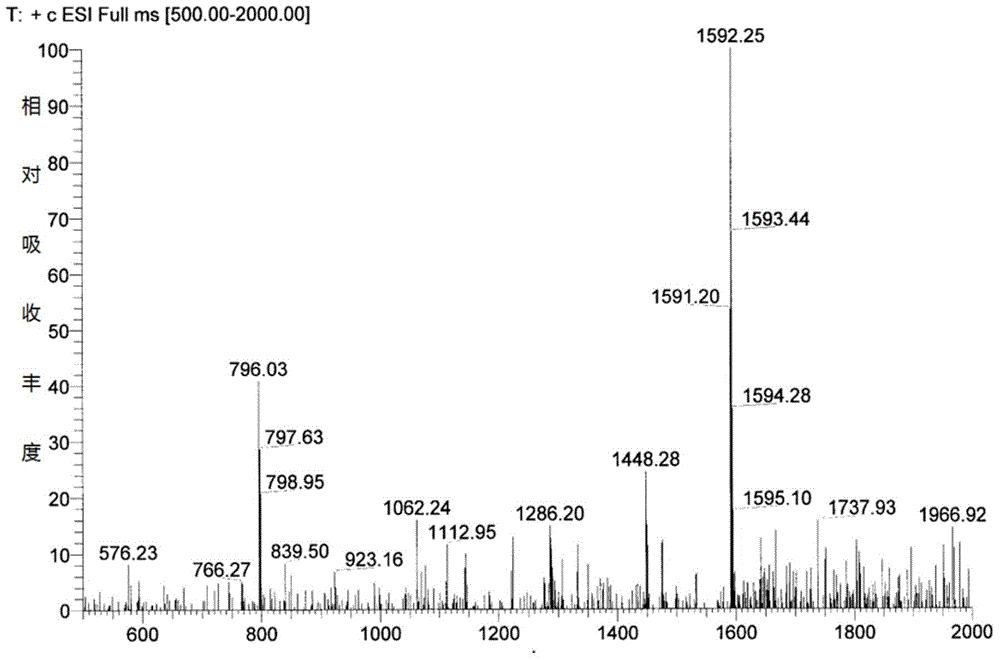
[0050] The preparation of embodiment 1 high-yield bacterial strain Cystobasidium deserticus HS807-AN-2396 (Kibdelosporangiumaridum)
[0051] The strain A.orientalis A82846 was isolated from soil samples in Haiti (for the strain A.orientalisA82846 see: Oliver Puk, Petra Huber, Daniel Bischoff, etc., Glycopeptide Biosynthesis in Amycolatopsis mediterranei DSM5908: Function of aHalogenase and a Haloperoxidase / Perhydrolase, Chemistry & Vol.9, 225–235, February, 2002), and then the HS807-O-13 strain was naturally isolated, and the HS807-O-13 strain was screened by mutagenesis to obtain the starting strain HS807-A-639. The bacterial suspension of HS807-A-639 was prepared and filtered with filter paper, and the cell dispersion degree of microscopic examination was over 95%, to ensure that most of them were single cells, and then used for mutagenesis treatment.
[0052] ARTP mutagenesis treatment: adjust the plasma generating gas-helium flow rate to 12.5L / min; the distance between the...
Embodiment 2
[0058] Morphological and culture characteristics of embodiment 2 bacterial strain HS807-AN-2396 (CGMCC No.10576)
[0059] The experiments were carried out with reference to the relevant contents in the "Streptomyces Identification Manual", "Classification and Identification of Actinomycetes", "Common Bacterial System Identification Manual" and "Molecular Cloning Experiment Guide".
[0060] Relevant symbols indicate explanation: 0: no growth; 1: weak growth; 2: able to grow, with a few spores; 3: good growth, with a large number of spores; 4: best growth, with abundant spores; +: positive; -: feminine.
[0061] Culture characteristics: Nine mediums of ISP1, ISP2, ISP3, ISP4, ISP5, Gaoshi No. 1, calcium malate, YMS and Cha's (obtained from Zhejiang Hisun Pharmaceutical Co., Ltd.) were used, and cultured at 28°C for 6~ After 8 days, observe the color and pigment situation of mycelia.
[0062] Table 1 Culture characteristics of bacterial strain HS807-AN-2396 on 9 kinds of media ...
Embodiment 3
[0065] Physiological and biochemical characteristics of Example 3 bacterial strain HS807-AN-2396 (CGMCC No.10576)
[0066] The culture conditions were all cultured at 28°C for 6-8 days.
[0067] a) Carbon source: ISP9 was used as the basal medium, and the final concentrations of various carbon sources were all 1.0%, and the stated percentages were mass percentages.
[0068] b) Inorganic nitrogen source: ISP9 was used as the basic medium, and the concentrations of potassium nitrate and ammonium sulfate were both 0.1%.
[0069] The utilization of carbon source and inorganic nitrogen source is shown in Table 2. The results of Table 2 show that strain HS807-AN-2396 has different degrees of utilization of different carbon sources, and can well utilize D-glucose, maltose, and D-lactose, but D-xylose, L-arabinose and glycine are not utilized. Ammonium nitrogen sources can be effectively utilized; however, nitrate nitrogen sources are difficult to utilize.
[0070] The utilization ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com