A preparation method of high-purity oritavancin key intermediate a82846b
An intermediate, high-purity technology, applied in the field of preparation of high-purity oritavancin key intermediate A82846B, can solve the problems of reduced yield, failure to adsorb, and inability to remove pigments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
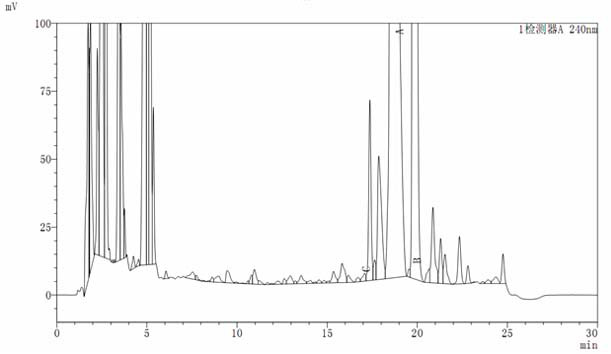
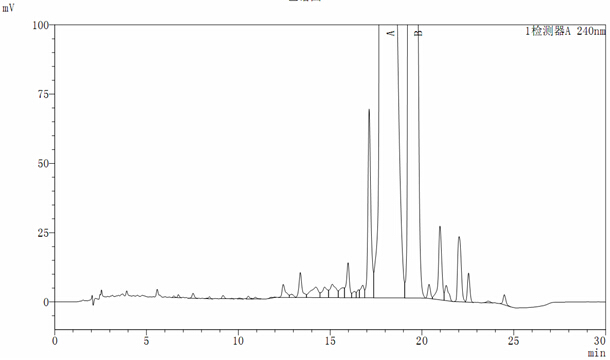
[0043] Oritavancin key intermediate A82846B was fermented in a 40L tank, and the fermentation unit was 650ug / ml (26g). The pH of the fermentation broth was adjusted to 10.5 with NaOH solution, stirred for 2 hours, and then centrifuged in a tube to obtain 36.8L of centrifuge (HPLC See atlas figure 1 ), the unit is 630ug / ml (23g). Adjust the pH of the centrifugate to 9.3, and then introduce it into HP20 adsorption resin (with a specification of Ф25*75), with a filling volume of 3000ml and a flow rate of 1BV / h. After purifying 9L of resin with water with a pH of 8, desorb it with 8L of 1% acetic acid aqueous solution at a flow rate of 2500ml / h. See figure 2 ), and then the desorbed mixture was concentrated by nanofiltration to a unit of 39102ug / ml (21.9g).
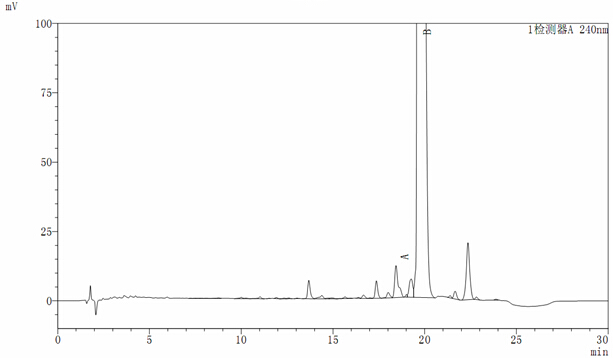
[0044] Introduce the concentrated solution into a well-balanced C18 column (with a specification of Ф25*75), and use acetonitrile: 0.5% NH 4 h 2 PO 4 =2:98 (v / v) for elution, the flow rate is 1500ml / h, and the componen...
Embodiment 2
[0051] The key intermediate of oritavancin A82846B was fermented in a 33L tank, and the fermentation unit was 340ug / ml (11.22g). The fermentation broth was adjusted to pH=10.3 with NaOH solution, stirred for 2 hours, and plate-and-frame pressure filtration was performed to obtain 35L of press-filtered liquid, the unit It is 310ug / ml (10.85g). The press filtrate was adjusted to pH=9.2, and then introduced into LX18 adsorption resin. After purifying 9L of resin with water with a pH of 8, use 8L of 0.5% acetic acid aqueous solution for desorption, and mix the components with higher concentration to form a desorption mixture, and then perform nanofiltration on the desorption mixture, and concentrate it to a unit of 35000ug / ml.
[0052] Introduce the concentrated solution into a well-balanced C18 column, and use the eluent (acetonitrile: 0.5% NH 4 h 2 PO 4 =3:97 (v / v)) to elute, collect components with a purity of more than 90%, and obtain a secondary desorption mixture. The s...
Embodiment 3
[0055] The key intermediate of oritavancin A82846B was fermented in 28.1L tank, and the fermentation unit was 420ug / ml (11.80g). The fermentation liquid was adjusted to pH=10.6 with NaOH solution, stirred for 2 hours and then centrifuged to obtain 25.5L centrifuge liquid, the unit was 432ug / ml (11.10g). Adjust the pH of the centrifuge to 9.5, divide the part into 4 equal volumes (5.5 L each, each containing 2.35 g of the product, and a purity of 25.69%), and introduce them into 4 regenerated and balanced resin columns (HP20, LX18, XAD1600, HZ816), then purify the resin column with 1L of purified water, and elute with 1.5% acetic acid aqueous solution. The components with higher concentration were collected to form a desorption mixture, which was sent for inspection, and the results of various purity tests were shown in Table 2:
[0056] Table 2: Primary purification effect of various resins
[0057] Resin name Resin load Loading amount The amount of product c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com