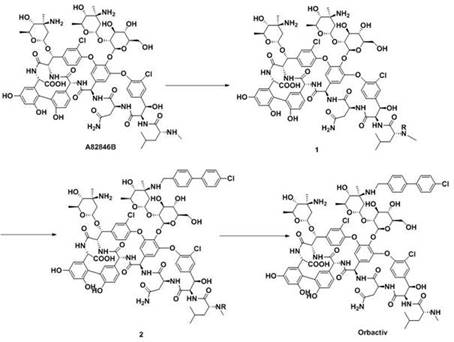
A kind of method for preparing oritavancin
An intermediate and amine-based technology, which is applied in the synthesis process of pharmaceutical intermediates and the field of preparation of oritavancin, can solve the problems that BrettpHos ligands are expensive and not suitable for industrialization, so as to reduce the generation of by-products and increase the yield and purity, the effect of improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
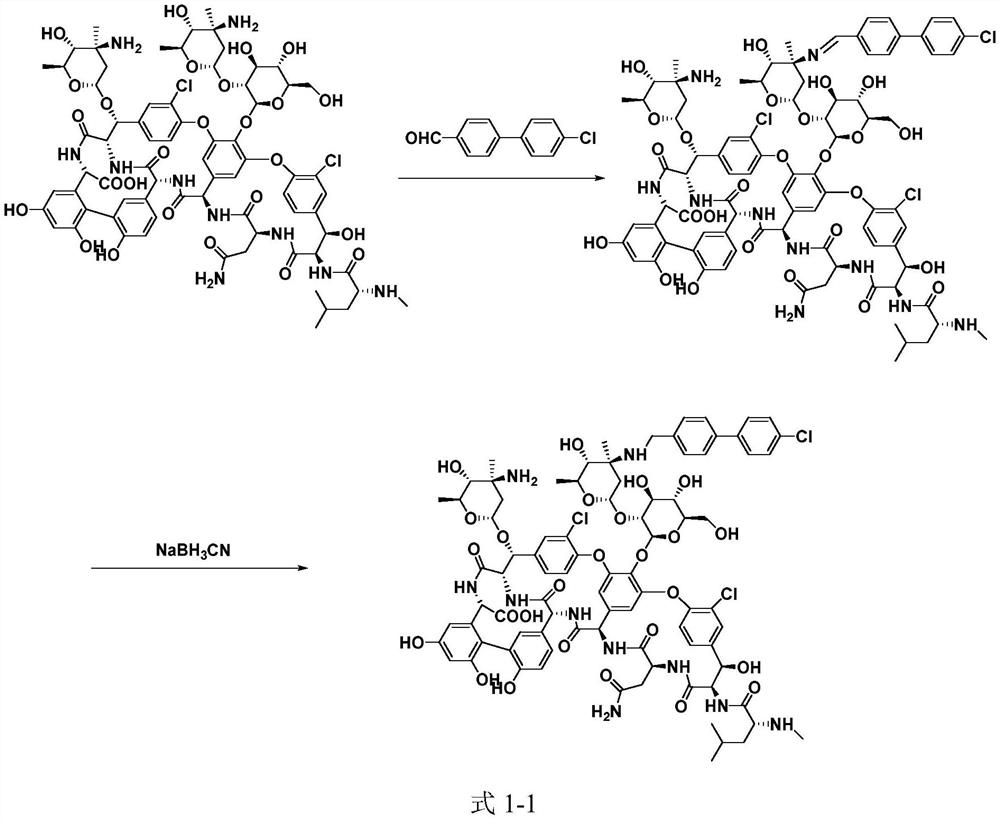
[0054] Synthesis of FMOC protecting group intermediate 3:
[0055]
[0056] 1) 330 mg of starting material A82846B was dissolved in 15 mL of H 2 In the mixed solvent of O / THF (1:1, V / V), add 0.1N NaOH dropwise to adjust pH=7, slowly add 10mL THF solution containing 101mg FmocOSu dropwise (add dropwise 0.1N NaOH to keep pH=7), 25~ React at 30°C for 6h;
[0057] 2) Add 50 mL of water to the reaction system, filter with suction, wash the solid with cold water at 4-10°C, dissolve the solid in 10 mL of DMSO, add 100 mL of acetone, filter with suction, wash the solid with acetone, and dry to obtain 327 mg, with a yield of 87%;
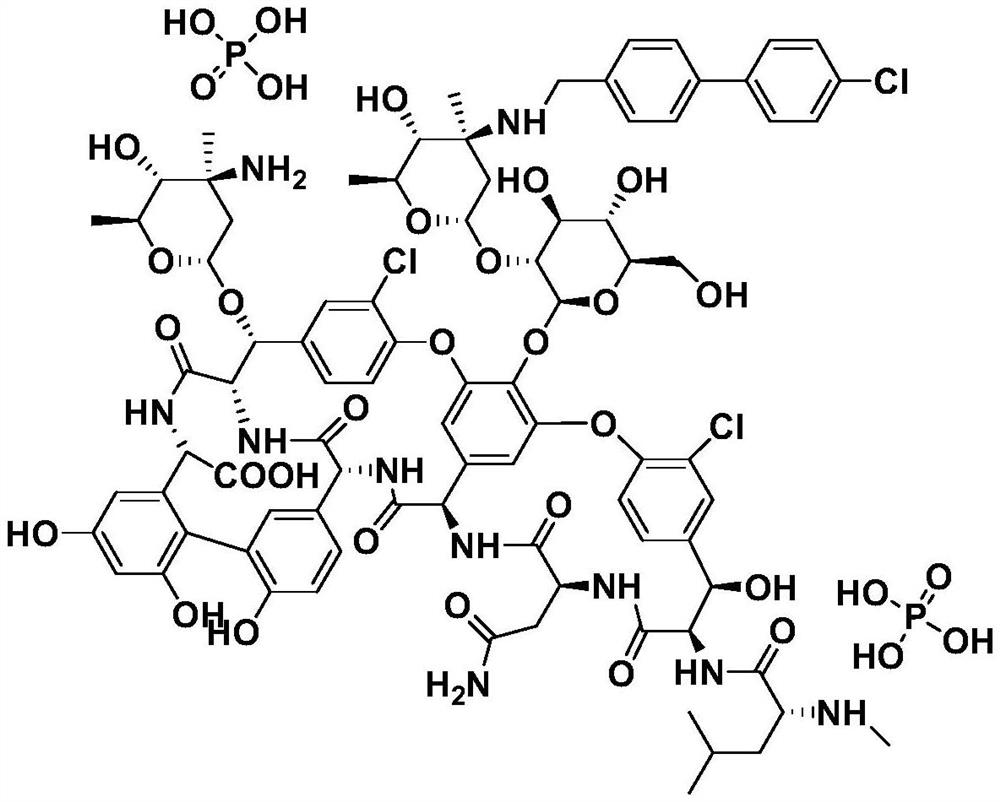
[0058] Synthesis of oritavancin:
[0059]
[0060] 3) Dissolve 300mg of intermediate compound 3 in 15mL of DMSO / DMF / MeOH (1:1:1, V / V / V) mixed solvent, add 54mg of 4'-chlorobiphenyl-4-carbaldehyde, 25-30°C Under stirring for 2h, slowly add 20mg NaBH in batches 3 CN, react at 25-30°C for 4h;
[0061] 4) Add 100 mL of acetone, filter with suction, an...
Embodiment 2
[0065] Synthesis of BOC protecting group intermediate 6:
[0066]
[0067] 1) 330 mg of starting material A82846B was dissolved in 15 mL of H 2 In the mixed solvent of O / dioxane (1:1, V / V), add 109mg Boc 2 O and 17 mg NaHCO 3 , react at 25-30°C for 6h;
[0068] 2) Add 50 mL of acetone to the reaction system, filter with suction, wash the solid with acetone, dissolve the solid in 10 mL of DMSO, add 100 mL of acetone, filter with suction, wash the solid with acetone, and dry to obtain 305 mg of intermediate compound 5 with a yield of 89%;
[0069] Synthesis of oritavancin:
[0070]
[0071] 3) Dissolve 300mg of intermediate compound 5 in 15mL of DMSO / DMF / MeOH (1:1:1, V / V / V) mixed solvent, add 54mg of 4'-chlorobiphenyl-4-carbaldehyde, 25-30°C Under stirring for 2h, slowly add 20mg NaBH in batches 3 CN, react at 25~30℃ for 4h;
[0072] 4) Add 100mL of acetone, filter with suction, and wash the solid with acetone; dry to obtain 307mg of brown powder of intermediate comp...
Embodiment 3
[0076] Synthesis of Cbz protecting group intermediate 7:
[0077]
[0078] 1) 330 mg of starting material A82846B was dissolved in 15 mL of H 2 In the mixed solvent of O / dioxane (1:1, V / V), add 103mg CbzOSu and 17mg NaHCO 3 , react at 25-30°C for 10h;
[0079] 2) Add 150 mL of acetone to the reaction system, filter with suction, wash the solid with acetone, dissolve the solid in 10 mL of DMSO, add 100 mL of acetone, filter with suction, wash the solid with acetone, and dry to obtain 311 mg of intermediate compound 7 with a yield of 87%;
[0080] Synthesis of oritavancin:
[0081]
[0082] 3) Dissolve 300mg of intermediate compound 7 in 15mL of DMSO / DMF / MeOH (1:1:1, V / V / V) mixed solvent, add 56mg of 4'-chlorobiphenyl-4-carbaldehyde, 25-30°C Under stirring for 2h, slowly add 21mg NaBH in batches 3 CN, react at 25~30℃ for 4h;
[0083] 4) Add 100 mL of acetone, filter with suction, wash the solid with acetone; dry to obtain 311 mg of brown powder of intermediate compoun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com