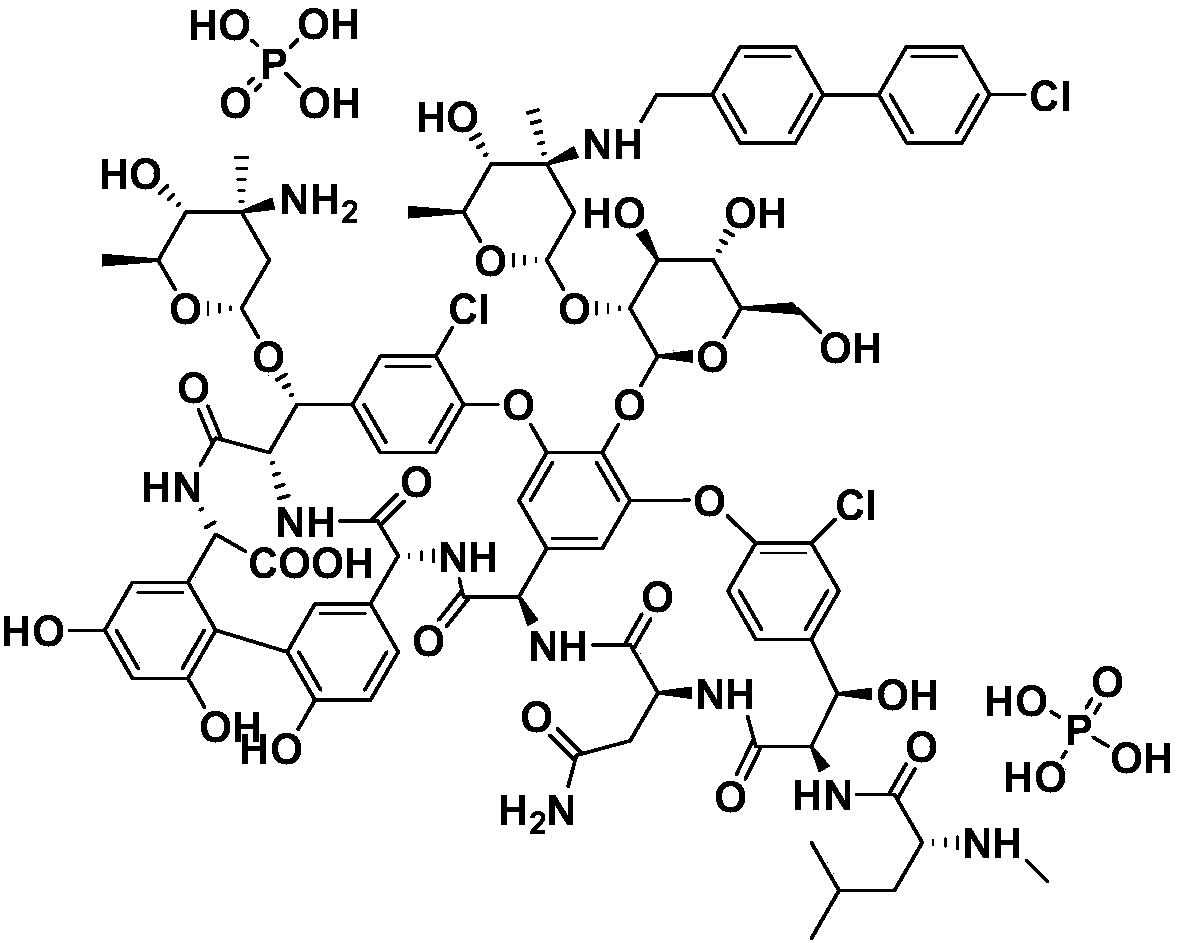
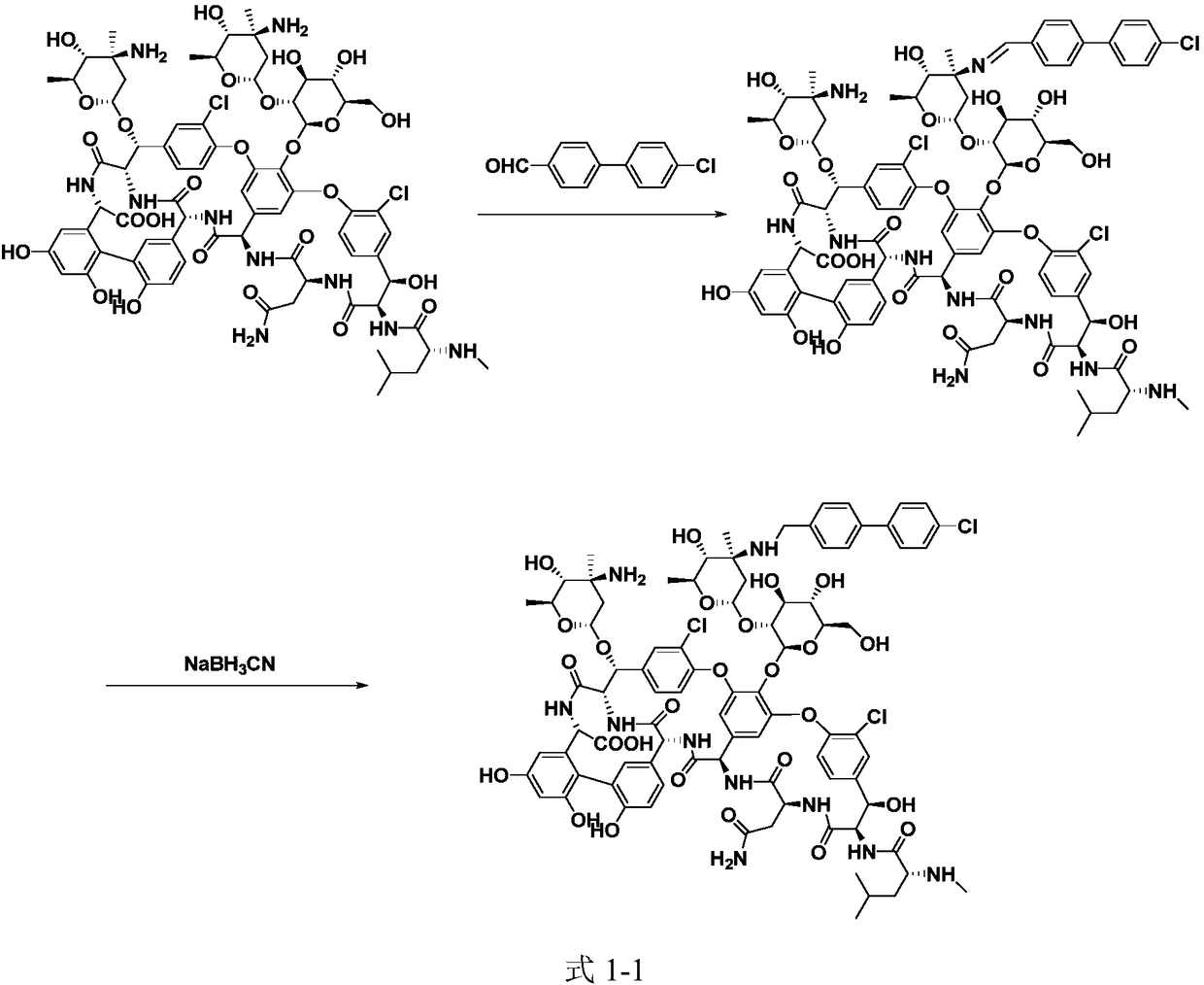
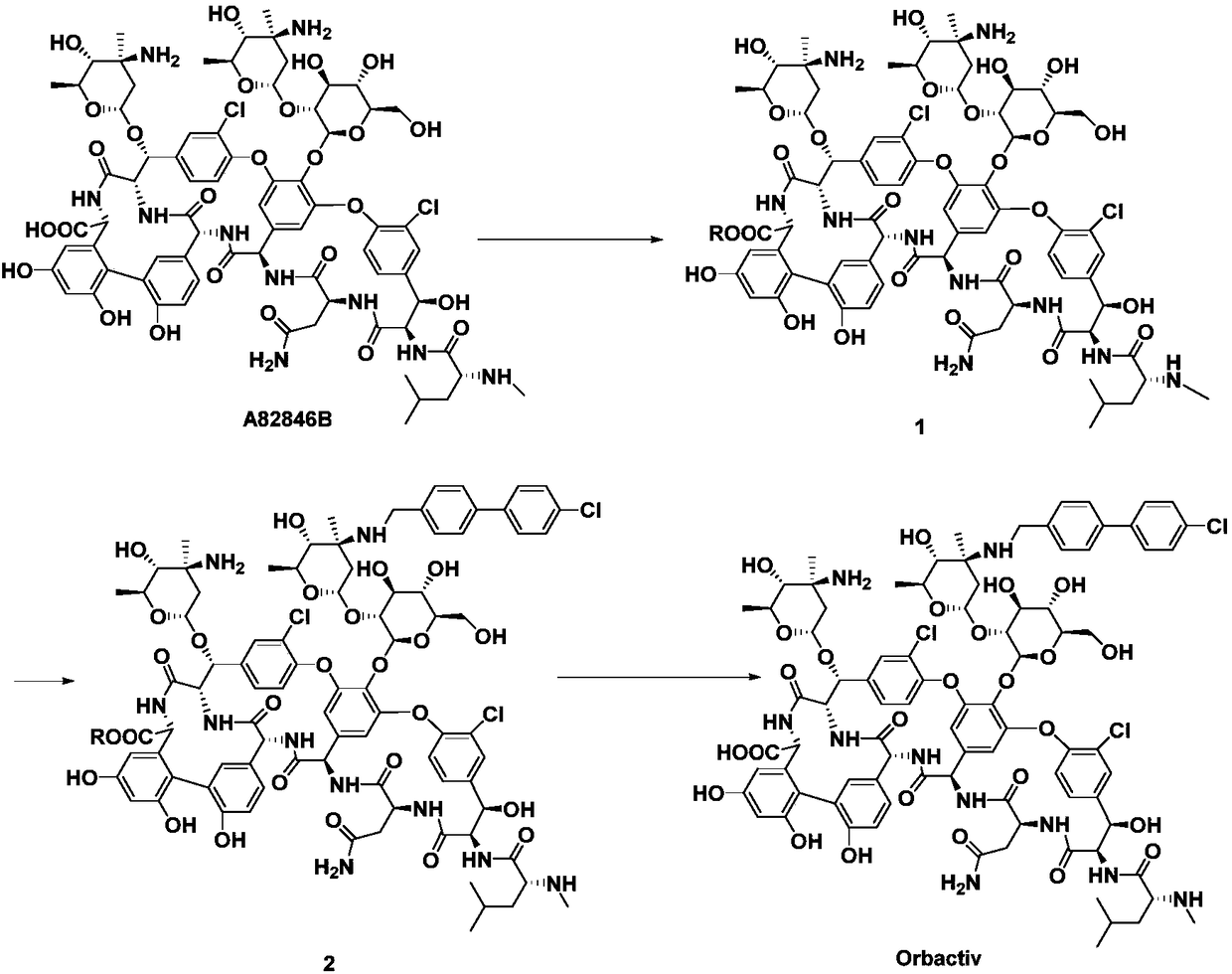
Method for preparing oritavancin by means of carboxyl protecting
A technology of carboxyl protecting group and carboxyl group, which is applied in peptide preparation methods, chemical instruments and methods, organic chemistry, etc., can solve the problems of unsuitability for industrialization and expensive price of BrettpHos ligands, so as to improve yield and purity and reduce side effects Product production, selectivity enhancement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Synthesis of Esterification Intermediate 3:
[0057]
[0058] 1) 200 mg of starting material A82846B was dissolved in 15 mL of CH 3 In OH solvent, add 0.02mL concentrated sulfuric acid dropwise, and react at 60°C for 3h;
[0059] 2) Add 50 mL of ether, filter with suction, and wash the solid with ether. Obtained 192mg powder, yield 95%;
[0060] Synthesis of oritavancin:
[0061]
[0062] 3) Dissolve 150mg of intermediate compound 3 in 15mL of DMSO / DMF / MeOH (1:1:1, V / V / V) mixed solvent, add 26mg of 4'-chlorobiphenyl-4-carbaldehyde, 20~50℃ Under stirring for 2h, slowly add 12mg NaBH in batches 3 CN, react at 25~30℃ for 5h;
[0063] 4) Add 100 mL of acetone, filter with suction, wash the solid with acetone, and dry to obtain 147 mg of brown powder of intermediate compound 4;
[0064] 5) Add intermediate 5 to 15 mL CH containing 10 mg NaOH 3 OH / H 2 In a mixed solvent of O (1:1, V / V), react at 60°C for 2h;
[0065] 6) Add glacial acetic acid dropwise to adjus...
Embodiment 2
[0067] Synthesis of esterified intermediate 5:
[0068]
[0069] 1) Dissolve 200mg of starting material A82846B and 23mg of p-nitrophenol in 15mL of DMSO solvent, add 0.02mL of concentrated sulfuric acid dropwise, and react at 100°C for 1h;
[0070] 2) Add 50 mL of diethyl ether, filter with suction, and wash the solid with diethyl ether; obtain 211 mg of powder with a yield of 97%;
[0071] Synthesis of oritavancin:
[0072]
[0073] 3) Dissolve 200mg of intermediate compound 5 in 20mL of DMSO / DMF / MeOH (1:1:1, V / V / V) mixed solvent, add 38mg of 4'-chlorobiphenyl-4-carbaldehyde, 20~50℃ Under stirring for 2h, slowly add 15mg NaBH in batches 3 CN, react at 25~30℃ for 4h;
[0074] 4) Add 150 mL of acetone, filter with suction, and wash the solid with acetone; dry to obtain 189 mg of brown powder of intermediate compound 6;
[0075] 5) Intermediate 5 was added to 20 mL CH containing 20 mg NaOH 3 OH / H 2 In a mixed solvent of O(1:1, V / V), react at 60°C for 2h.
[0076] 6...
Embodiment 3
[0078] Synthesis of esterified intermediate 7:
[0079]
[0080] 1) 150 mg of starting material A82846B was dissolved in 15 mL (CH 3 ) 2 In CHOH solvent, add 0.02mL concentrated sulfuric acid dropwise, and react at 75-80°C for 2h;
[0081] 2) Add 50 mL of diethyl ether, filter with suction, and wash the solid with diethyl ether; 149 mg of powder is obtained, with a yield of 97%;
[0082] Synthesis of oritavancin:
[0083]
[0084] 3) 120mg of intermediate compound 7 was dissolved in 15mL DMSO / DMF / (CH 3 ) 2 In the mixed solvent of CHOH (1:1:1, V / V / V), add 24mg 4'-chlorobiphenyl-4-carbaldehyde, stir at 20~50℃ for 2h, slowly add 10mg NaBH in batches 3 CN, react at 25~30℃ for 4h;
[0085] 4) Add 100 mL of acetone, filter with suction, and wash the solid with acetone; dry to obtain 118 mg of brown powder of intermediate compound 8;
[0086] 5) Add intermediate 5 to 15 mL CH containing 10 mg NaOH 3 OH / H 2 In a mixed solvent of O (1:1, V / V), react at 60°C for 2h;
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com