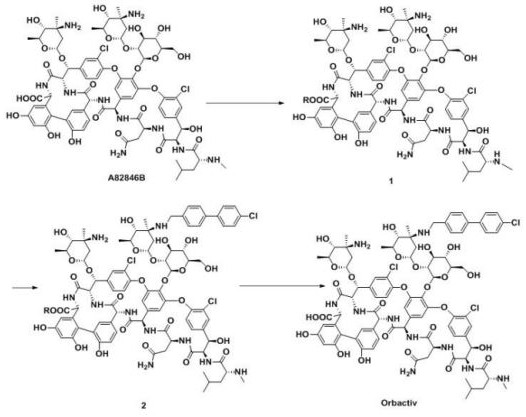
A kind of method for carboxyl protection preparation oritavancin
A carboxyl-protecting group and carboxyl-based technology, applied in peptide preparation methods, chemical instruments and methods, bulk chemical production, etc., can solve the problems that BrettpHos ligands are expensive and unsuitable for industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Synthesis of Esterification Intermediate 3:
[0056]
[0057] 1) 200mg starting material A82846B dissolved in 15mL CH 3 In OH solvent, add 0.02mL concentrated sulfuric acid dropwise, and react at 60°C for 3h;
[0058] 2) Add 50 mL of ether, filter with suction, and wash the solid with ether. Obtain 192mg powder, yield 95%;
[0059] Synthesis of oritavancin:
[0060]
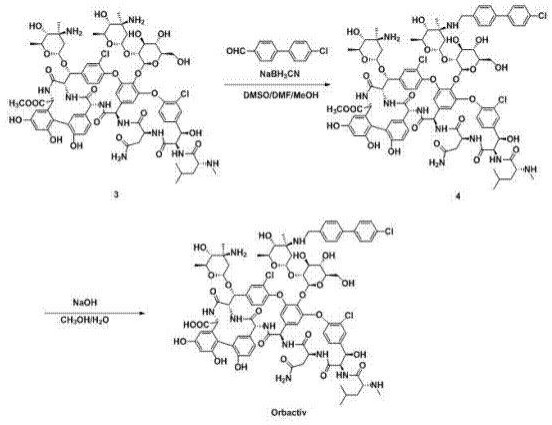
[0061] 3) 150 mg of intermediate compound 3 was dissolved in 15 mL of a mixed solvent of DMSO / DMF / MeOH (1:1:1, V / V / V), and 26 mg of 4'-chlorobiphenyl-4-carbaldehyde was added, 20-50 Stir at ℃ for 2h, slowly add 12mg NaBH in batches 3 CN, react at 25-30°C for 5 h;
[0062] 4) Add 100 mL of acetone, filter with suction, wash the solid with acetone, and dry to obtain 147 mg of brown powder of intermediate compound 4;
[0063] 5) Add intermediate 5 to 15 mL CH containing 10 mg NaOH 3 OH / H 2 In a mixed solvent of O (1:1, V / V), react at 60°C for 2h;
[0064] 6) Add glacial acetic acid dropwise to ...
Embodiment 2
[0066] Synthesis of esterified intermediate 5:
[0067]
[0068] 1) Dissolve 200 mg of starting material A82846B and 23 mg of p-nitrophenol in 15 mL of DMSO solvent, add 0.02 mL of concentrated sulfuric acid dropwise, and react at 100 °C for 1 h;
[0069] 2) Add 50 mL of diethyl ether, filter with suction, and wash the solid with diethyl ether; 211 mg of powder is obtained, with a yield of 97%;
[0070] Synthesis of oritavancin:
[0071]
[0072] 3) 200 mg of intermediate compound 5 was dissolved in 20 mL of a mixed solvent of DMSO / DMF / MeOH (1:1:1, V / V / V), and 38 mg of 4'-chlorobiphenyl-4-carbaldehyde was added, 20-50 Stir at ℃ for 2h, slowly add 15mg NaBH in batches 3 CN, react at 25-30°C for 4 h;
[0073] 4) Add 150mL of acetone, filter with suction, wash the solid with acetone; dry to obtain 189mg of brown powder of intermediate compound 6;
[0074] 5) Add intermediate 5 to 20 mL CH containing 20 mg NaOH 3 OH / H 2 In a mixed solvent of O (1:1, V / V), react at 60°C...
Embodiment 3
[0077] Synthesis of esterified intermediate 7:
[0078]
[0079] 1) Dissolve 150mg of starting material A82846B in 15mL (CH 3 ) 2 In CHOH solvent, add 0.02mL concentrated sulfuric acid dropwise, and react at 75-80°C for 2h;
[0080] 2) Add 50 mL of diethyl ether, filter with suction, and wash the solid with diethyl ether; 149 mg of powder is obtained, with a yield of 97%;
[0081] Synthesis of oritavancin:
[0082]
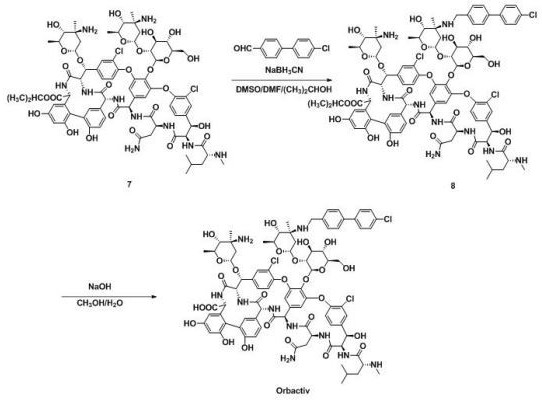
[0083] 3) 120mg of intermediate compound 7 was dissolved in 15mL DMSO / DMF / (CH 3 ) 2 In the mixed solvent of CHOH (1:1:1, V / V / V), add 24 mg 4'-chlorobiphenyl-4-carbaldehyde, stir at 20-50°C for 2 hours, slowly add 10 mg NaBH in batches 3 CN, react at 25-30°C for 4 h;
[0084] 4) Add 100 mL of acetone, filter with suction, and wash the solid with acetone; dry to obtain 118 mg of brown powder of intermediate compound 8;
[0085] 5) Add intermediate 5 to 15 mL CH containing 10 mg NaOH 3 OH / H 2 In a mixed solvent of O (1:1, V / V), react at 60°C for 2h;
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com