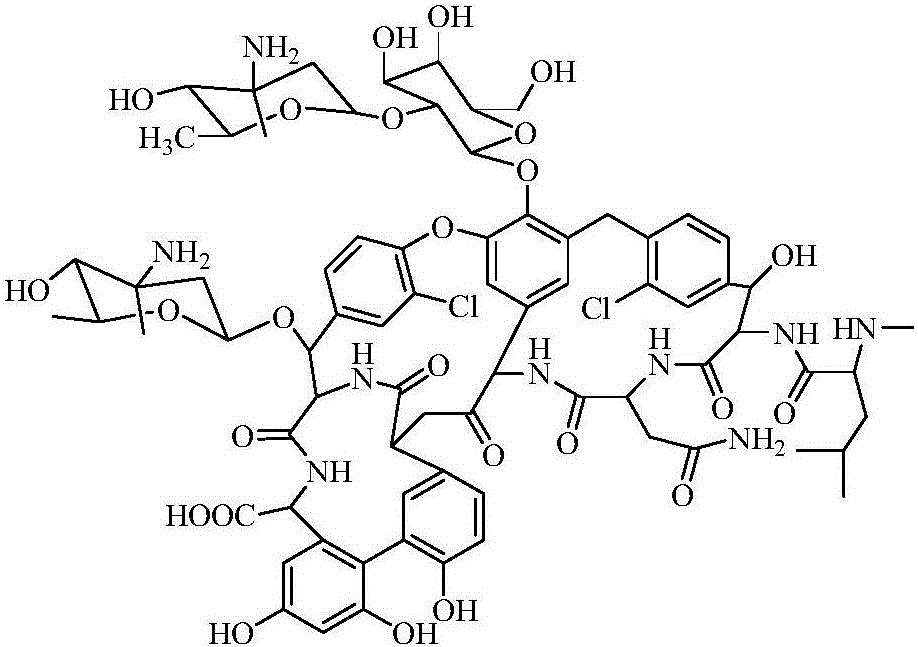
Purification method of Oritavancin intermediate A82846B
A purification method and technology of oritavancin, which are applied in the preparation methods of peptides, chemical instruments and methods, organic chemistry, etc., can solve the problems of inability to provide high-purity oritavancin intermediates, etc., and achieve simple operation and solvent use. Low volume and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
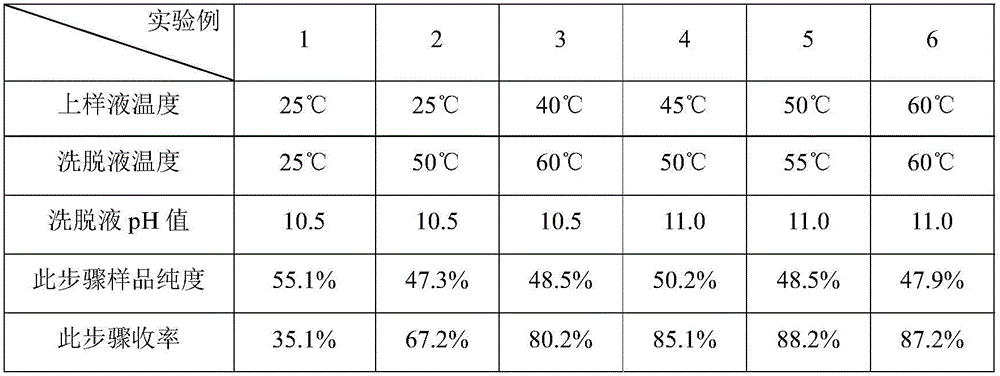
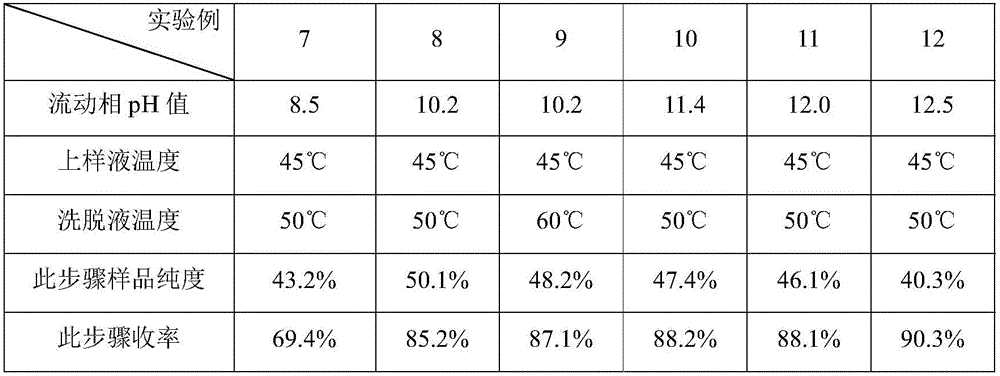
[0042] Embodiment 1: solid-liquid separation and cation exchange resin pretreatment
[0043] 300L of A82846B fermented liquid (according to the technology disclosed in CN87106483A is fermented to obtain the fermented liquid, the bacterial strain used is Kibdelosporangium aridum (Pseudocystis) NRRL 18098, microfiltration is carried out with a ceramic membrane with a membrane pore size of 0.1-0.2 μm, water replenishment cycle , Discard the bacteria residue, collect the filtrate 900 L. The detection unit of A82846B is 0.47g / L, and the total amount is 423.0g.
[0044] Adjust the pH of 900L of the clarified liquid to 7.0 and purify it with a 60L FPC22 resin column, and then use 2BV of purified water, 2BV of 70% ethanol to wash and 3BV of 50mM ammonia solution to obtain 180L of A82846B eluent with a purity of 51.9%.
Embodiment 2
[0045] Embodiment 2: solid-liquid separation and cation exchange resin pretreatment
[0046] Take 320L of A82846B fermentation broth, use a plate frame filter cake and wash it with water, discard the filter cake, and collect 350L of filtrate. The detection unit of A82846B was 1.25g / L, and the total amount was 437.5g.
[0047] Adjust the pH of 350L of the filtrate to 7.0 and purify it with a 60L HD-8 resin column, and then wash it with 2BV of purified water, 3BV of 50% ethanol and 2BV of 150mM ammonia solution to obtain 120L of A82846B eluate with a purity of 41.1%.
Embodiment 3
[0048] Embodiment 3: solid-liquid separation and cation exchange resin pretreatment
[0049] Take 320L of A82846B fermentation broth, use a plate frame filter cake and wash it with water, discard the filter cake, and collect 340L of filtrate. The detection unit of A82846B was 1.36g / L, and the total amount was 462.4g.
[0050] Adjust the pH of 600L of the filtrate to 7.0 and purify it with a 60L HD-8 resin column, and then use 2BV of purified water, 3BV of 80% ethanol to wash impurities and 2BV of 100mM sodium hydroxide aqueous solution to obtain 110L of A82846B elution with a purity of 50.2%. .
[0051] Based on Examples 1 and 2, during the sample elution process, the concentration of the elution phase (ammonia solution) used will affect the purity of related substances in the A82846B eluent at this step. Generally speaking, we will use more than 45% of A82846B The eluate is processed in the next step, and finally A82846B with better purity can be obtained, and at the same t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com