Producing strain of oritavancin intermediate and application of producing strain
A technology of oritavancin and intermediates, which is applied in the direction of bacteria, microorganisms, and methods based on microorganisms, can solve the problems that cannot meet the needs of scientific research and actual production, the difficulty of separating target products, and the low probability of natural mutations. Improve the yield of the extraction step, reduce the cost of fermentation and extraction, and reduce the effect of extraction cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
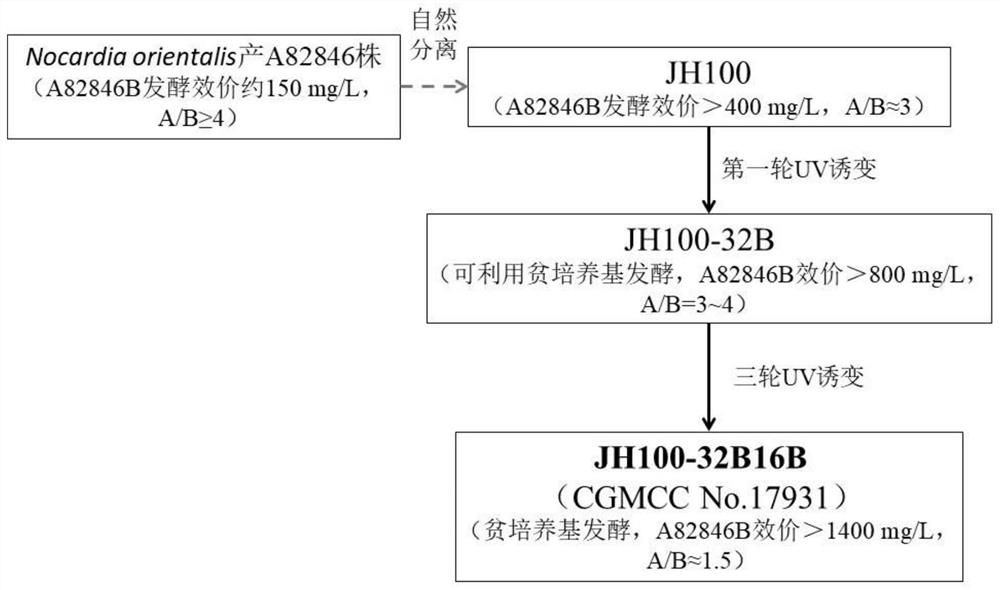
[0069] Obtainment of strain JH100-32B16B (CGMCC No.17931).
[0070] JH100 strain was obtained by natural isolation from a strain of Nocardia orientalis producing A82846 series compounds (Hamill et al. A82846 ANTIBIOTICS [P]. USP 5312738, May 17, 1994). Taking JH100 as the starting strain, the subsequent mutagenesis breeding operation was started.
[0071]Cell preparation: First, dilute the JH100 liquid culture with sterile water, add glass beads into the dilution test tube, shake fully on the shaker to ensure that the hyphae are dispersed; use a sterile graduated pipette to draw about 0.1mL of appropriate dilution The bacterial suspension was added dropwise on a sterile ISP2 plate, and coated with an L-shaped glass spatula. Prepare several coating plates by following this procedure.
[0072] The first round of mutagenesis: the above-mentioned starting strain (JH100) coated plate was placed under ultraviolet light irradiation. The parameters of the ultraviolet lamp irradiati...
Embodiment 2
[0080] Identification of strain JH100-32B16B (CGMCC No.17931).
[0081] (1) Morphological characteristics of strain JH100-32B16B
[0082] After culturing the strain JH100-32B16B on solid media (plates) with different formulations at 28°C for 5-7 days, observe the colony morphology, see Table 1:
[0083] Table 1
[0084]
[0085] The aerial hyphae of strain JH100-32B16B were picked and observed under an optical microscope. The characteristics were: spore filaments were straight, flexible, hooked, loose and tight spiral; spores were cylindrical and had cystic structures.
[0086] (2) Culture and physiological and biochemical characteristics of strain JH100-32B16B
[0087] The physiological and biochemical characteristics of the strain JH100-32B16B were investigated during the culture process. Table 2 shows the carbon source utilization characteristics; Table 3 shows the nitrogen source utilization characteristics of the strain; Table 4 shows other physiological and biochemi...
Embodiment 3
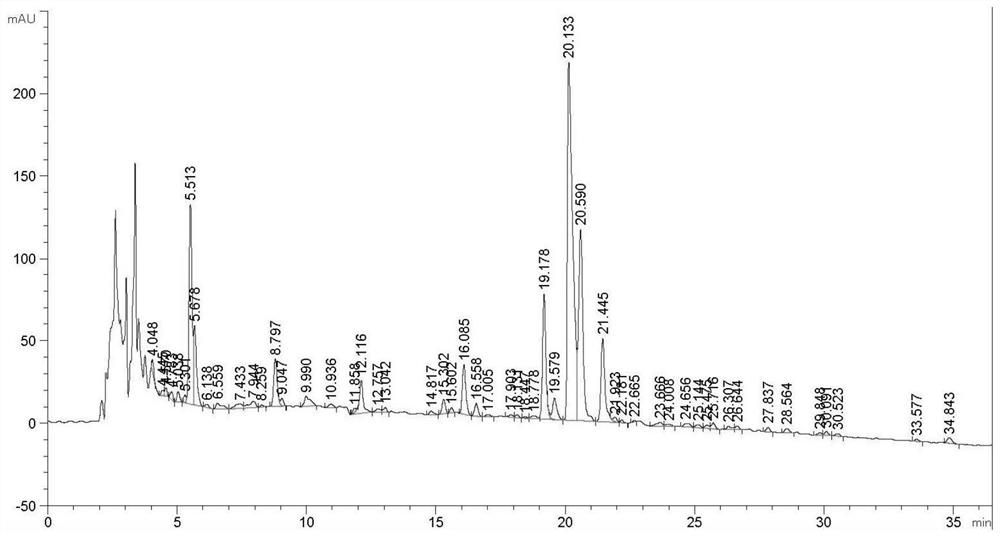
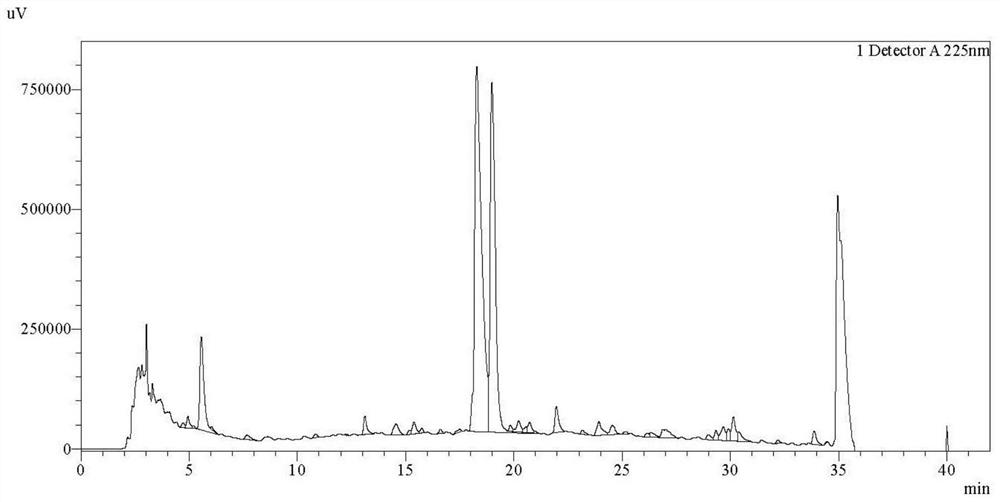
[0102] Shake flask fermentation of control strain JH100. The JH100 strains preserved in glycerol tubes were transferred to the ISP-2 slant, and placed in a constant temperature and humidity incubator at 28°C for activation and culture for 5-7 days. Pick the activated bacterial lawn and transfer it to the seed bottle. The liquid volume of the seed bottle is: 30mL seed medium in a 250mL triangular flask. The formula of the seed medium is (g / L): glucose 15, maltodextrin 15, hydrolyzed casein 3, yeast extract 3, pH 7.2. The seed bottle was shaken and cultivated on a constant temperature shaker at 28° C. for 48 hours, and the shaker rotated at 200 rpm. The mature seeds were transferred to fermentation shake flasks for shake flask fermentation. The shake flask fermentation medium formula is (g / L): glucose 30, maltodextrin 30, molasses 25, hydrolyzed casein 10, yeast extract 10, cottonseed cake powder 10, pH 7.5. The amount of liquid in the fermentation bottle is 20mL medium in a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fermentation titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com