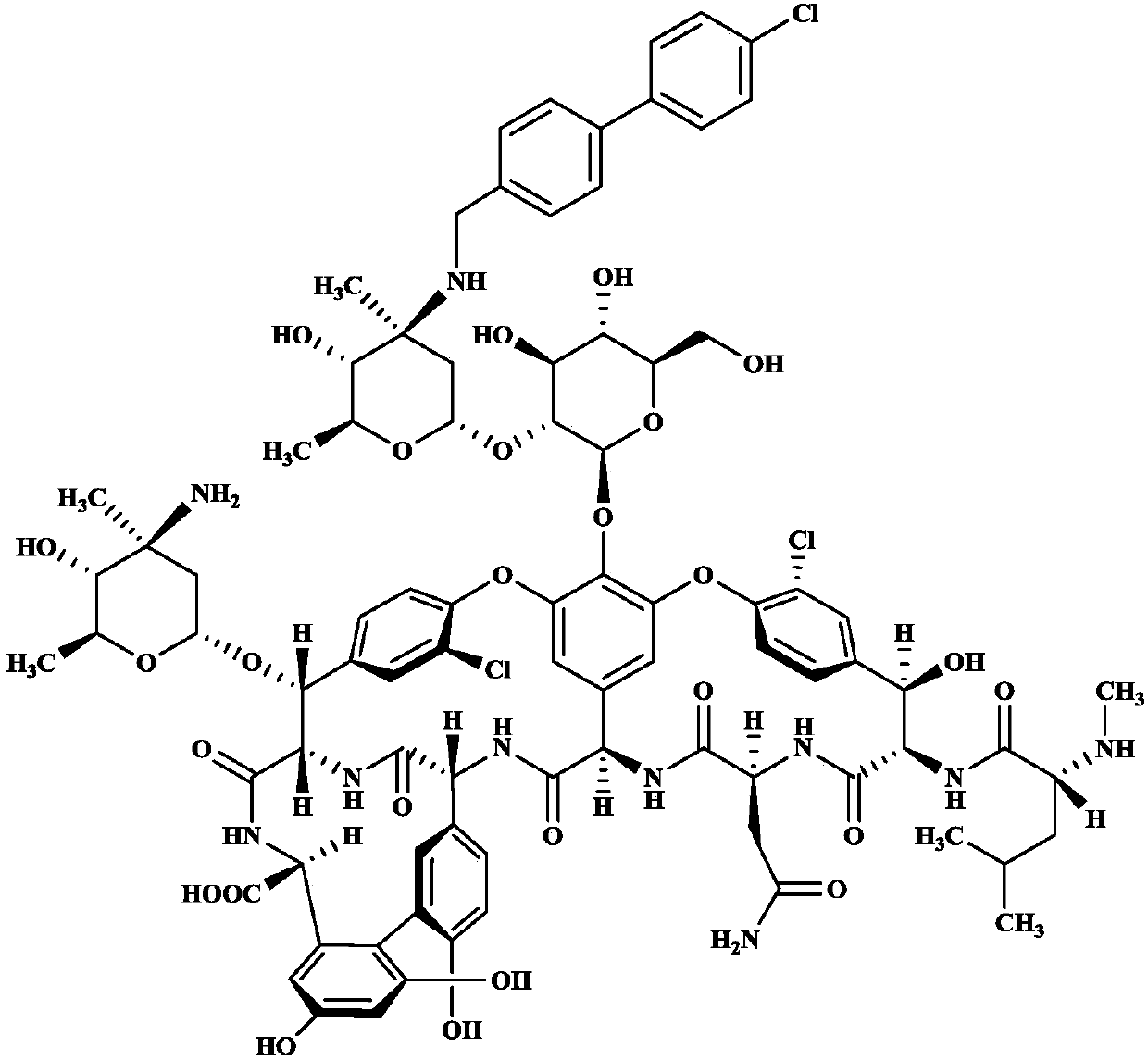
Oritavancin purification method
A purification method and polymer technology, applied in peptide preparation methods, chemical instruments and methods, organic chemistry, etc., to achieve the effects of reducing impurity content, improving product quality, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Utilizing Uni PSN to prepare high-purity oritavancin finished product
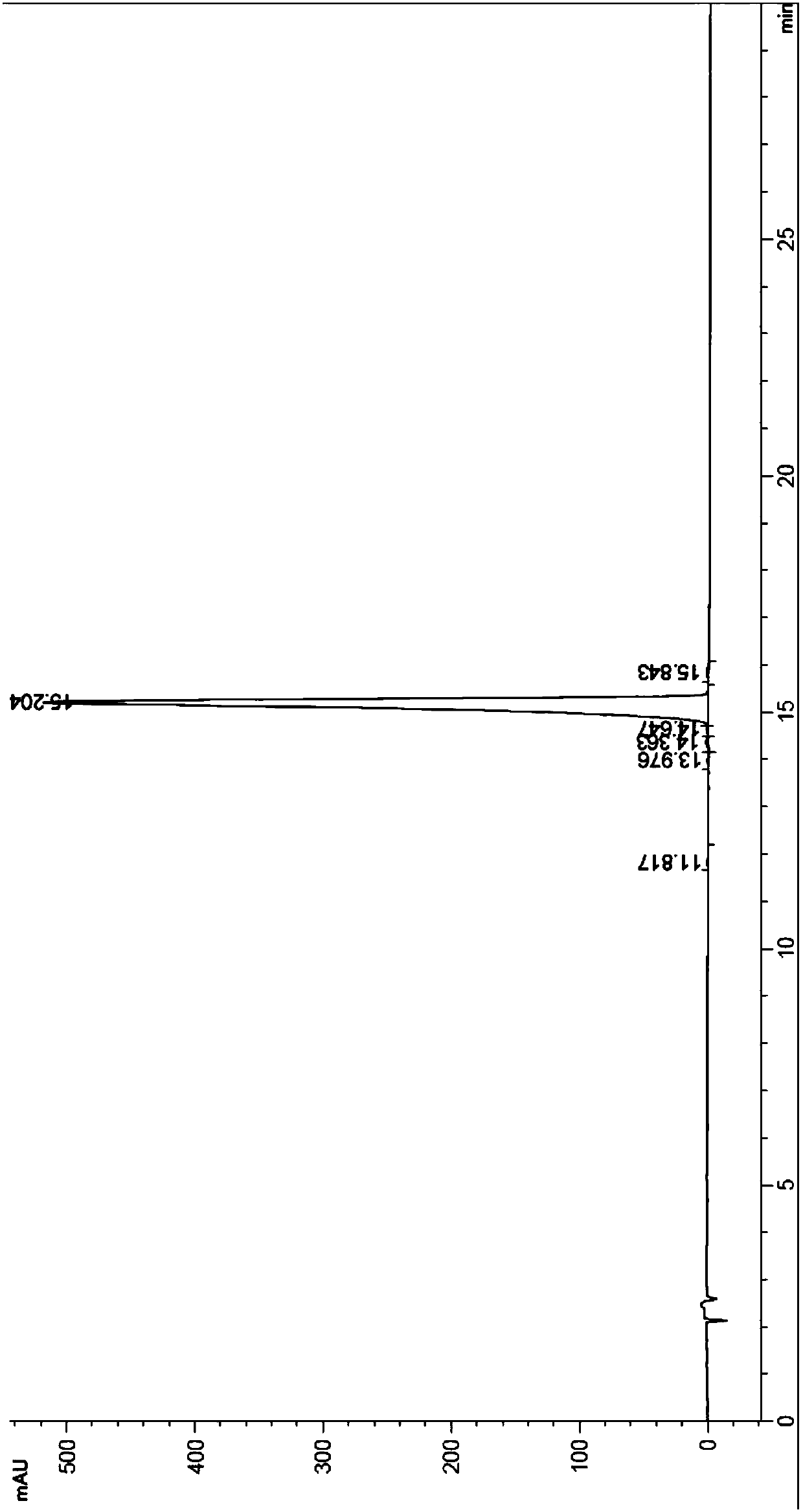
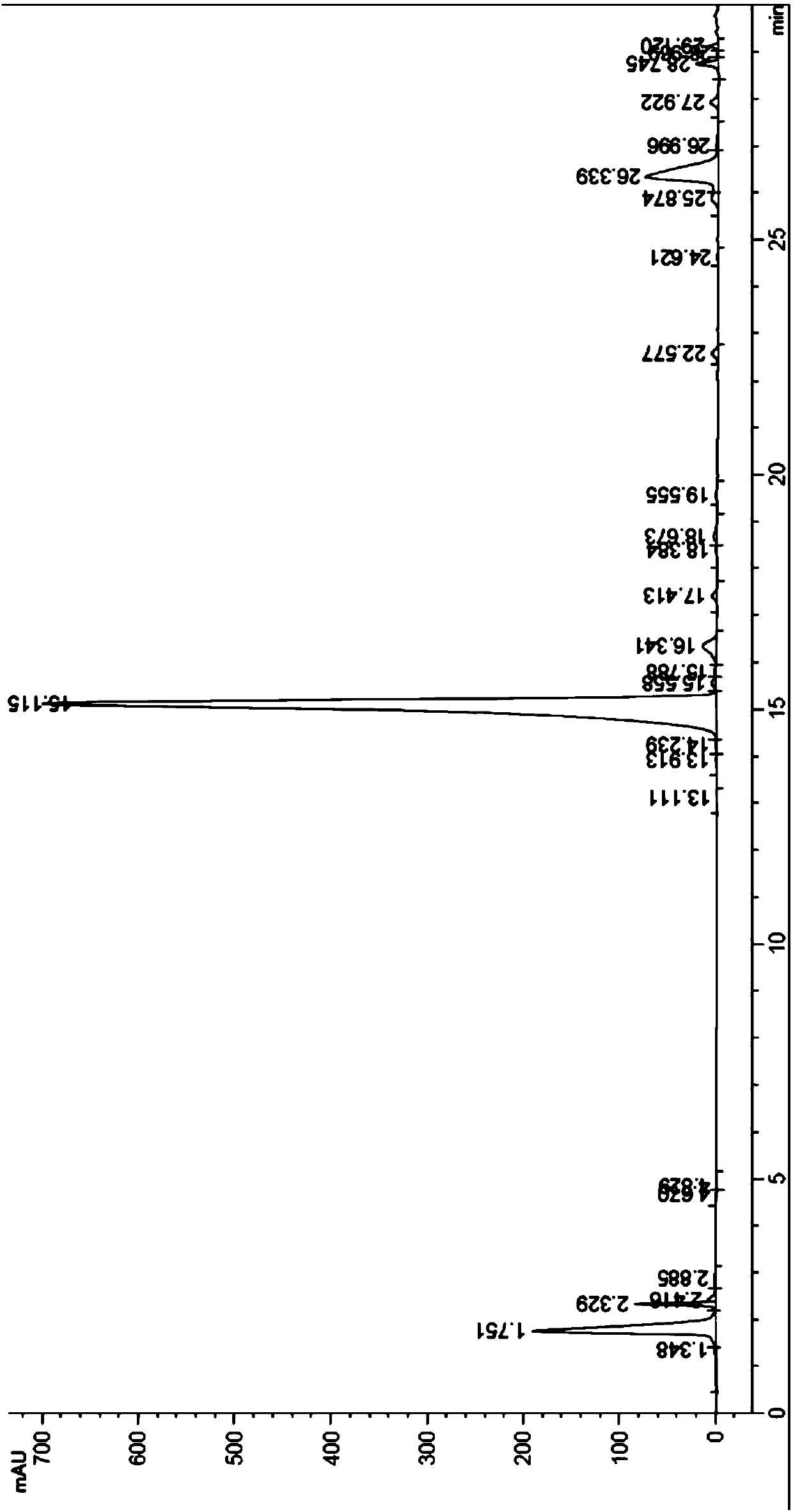
[0039] Take 80ml Uni PSN 40-300 polymer microspheres and pack them into a column. The column size is 1.8cm*50cm. After the microspheres settle freely, use 300ml 0.5% NaOH aqueous solution: acetonitrile=1:1 (volume ratio) to regenerate the microspheres. The column was equilibrated with 300 ml of 5% acetonitrile in water. Get oritavancin crude product 3.2g, add 100ml 5% acetonitrile aqueous solution, adjust pH value to 2.1 with 4M HCl, shake and dissolve, the chromatographic content of oritavancin in the crude product solution is 70.1% (such as figure 2 Shown), sample loading, sample loading speed is 300ml / hr. Pre-wash with 200ml of 10% acetonitrile aqueous solution to remove unreacted raw material A82846B; 2 PO 4 , phosphoric acid to adjust the pH value to 3.3) gradient elution, the elution rate is 400ml / hr, 80ml / bottle collects fractions, and HPLC detects the content and purity of t...
Embodiment 2
[0041] Example 2: Utilizing Uni PS to prepare high-purity oritavancin finished product
[0042] Take 80ml Uni PS 40-300 polymer microspheres and pack them into a column. The chromatographic column specification: 1.8cm*50cm. After the microspheres settle freely, use 300ml 0.5% NaOH aqueous solution: acetonitrile=1:1 (volume ratio) to regenerate the microspheres. The column was equilibrated with 300 ml of 5% acetonitrile in water. Take 2.0g of oritavancin crude product, add 100ml of 5% acetonitrile aqueous solution, adjust the pH value to 2.1 with 4M HCl, shake and dissolve, the chromatographic content of oritavancin in the crude solution is 70.3%, and load the sample at a speed of 300ml / hr. Pre-wash with 200ml of 10% acetonitrile aqueous solution to remove unreacted raw material A82846B; 2 PO 4 , phosphoric acid to adjust the pH value to 3.3) gradient elution, the elution rate is 400ml / hr, 80ml / bottle collects fractions, and HPLC detects the content and purity of the main...
Embodiment 3
[0044] Example 3: Utilizing Uni PMM to prepare high-purity oritavancin finished product
[0045]Take 80ml Uni PMM 40-300 polymer microspheres and pack them into a column. The chromatographic column specification: 1.8cm*50cm. After the microspheres settle freely, use 300ml 0.5% NaOH aqueous solution: acetonitrile=1:1 (volume ratio) to regenerate the microspheres. The column was equilibrated with 300 ml of 5% acetonitrile in water. Take 2.0g of oritavancin crude product, add 100ml of 5% acetonitrile aqueous solution, adjust the pH value to 2.1 with 4M HCl, shake and dissolve, the chromatographic content of oritavancin in the crude solution is 70.3%, load the sample, and the sample loading speed is 300ml / hr. Pre-wash with 200ml of 10% acetonitrile aqueous solution to remove unreacted raw material A82846B; 2 PO 4 , phosphoric acid to adjust the pH value to 3.3) gradient elution, the elution rate is 400ml / hr, 80ml / bottle collects fractions, and HPLC detects the content and pu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com