A kind of preparation method of oritavancin
A technology of raw materials and acetate, applied in the direction of peptides, etc., can solve the problems of cumbersome reaction operations and post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
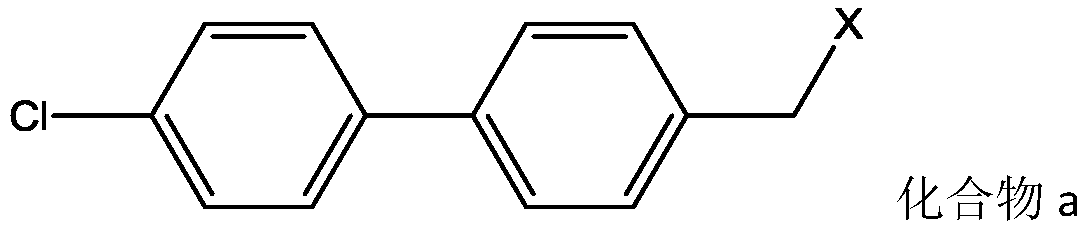
[0021] N 2 Under protection, Brettpho catalyst (10.1mg, 1.26×10 -2 mmol), Brettphos ligand (6.77mg, 1.26×10 -2 mmol), 4-(bromomethyl)-4'-biphenyl chloride (0.142g, 0.504mmol), A82846B acetate (1.0g, 0.605mmol), potassium tert-butoxide (0.27g, 2.42mmol) and 1 , 4-dioxane 10ml. The reaction mixture was heated to 100°C and kept for 8 hours. After the reaction was completed, the reaction solution was filtered to remove insoluble matter, and the obtained filtrate was purified by Preparative HPLC. The preparation solution was desalted and concentrated in vacuo to remove the organic solvent, and freeze-dried to obtain 0.769 g of the final product oritavancin, with a yield of 70.88%.
Embodiment 2
[0023] N 2 Under protection, Brettphos catalyst (10.1mg, 1.26×10 -2 mmol), Brettphos ligand (6.77mg, 1.26×10 -2 mmol), 4-(chloromethyl)-4'-biphenyl chloride (0.120g, 0.504mmol), A82846B acetate (1.0g, 0.605mmol), potassium tert-butoxide (0.27g, 2.42mmol) and toluene 10ml. The reaction mixture was heated to 100°C and kept for 10 hours. After the reaction was completed, the reaction solution was filtered to remove insoluble matter, and the obtained filtrate was purified by Preparative HPLC. The preparation solution was desalted and concentrated in vacuo to remove the organic solvent, and freeze-dried to obtain 0.725 g of the final product oritavancin, with a yield of 66.82%.
Embodiment 3
[0025] N 2 Under protection, Brettphos catalyst (10.1mg, 1.26×10 -2 mmol), Brettphos ligand (6.77mg, 1.26×10 -2 mmol), 4-(bromomethyl)-4'-biphenyl chloride (0.142g, 0.504mmol), A82846B acetate (1.0g, 0.605mmol), sodium tert-butoxide (0.23g, 2.42mmol) and 1 , 4-dioxane 10ml. The reaction mixture was heated to 100°C and kept for 8.5 hours. After the reaction was completed, the reaction solution was filtered to remove insoluble matter, and the obtained filtrate was purified by PreparativeHPLC. The preparation solution was desalted and concentrated in vacuo to remove the organic solvent, and freeze-dried to obtain 0.756 g of the final product oritavancin, with a yield of 69.68%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com