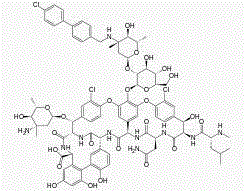
Stable oritavancin compound
An oritavancin and compound technology, which is applied in the field of medicine, can solve the problems of a large number of oritavancin impurities and a high total amount of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
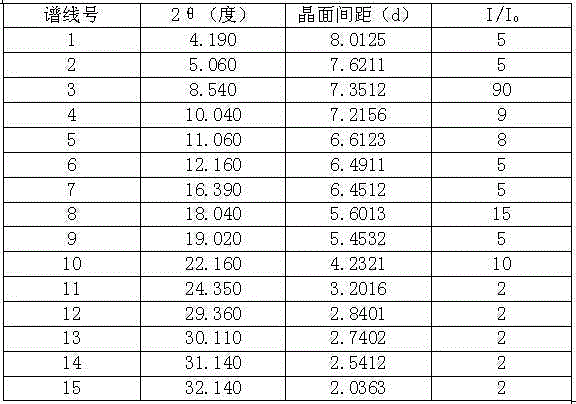
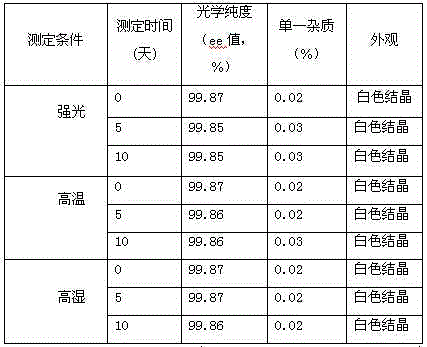
[0031] In a 3000ml reaction bottle equipped with stirring, thermometer and condenser, add 300g oritavancin and 2700ml of acetone-water (2:4.5) mixture, start stirring, heat up to 75°C, wait until all dissolve, Strain while hot. Cool down to 35°C and keep warm for 3 hours; then cool down to 25°C and keep warm for 4 hours to precipitate crystals, filter, and dry to obtain 276.1 g of high-purity oritavancin compound crystals with an optical purity of 99.88%. The detection of solvent residues meets the requirements.
[0032] Microparticles or microspheres are prepared by combining the compounds of the present invention with a pharmaceutically acceptable solid or liquid carrier, and optionally with pharmaceutically acceptable adjuvants and vehicles, using standard and conventional techniques. The composition is used for the preparation of oral preparations. It is given by way of example only and in no way is it intended to limit the scope of the invention in any way.
Embodiment 2
[0034] Tablets containing the oritavancin compound
[0035] Prescription: 300 grams of oritavancin compound, 50 grams of lactose, 260 grams of microcrystalline cellulose, 50 grams of sodium carboxymethyl starch, 5 grams of magnesium stearate, appropriate amount of distilled water, made into 1000 tablets.
[0036] Process: crush the oritavancin compound, pass through a 60-mesh sieve, mix with other materials, use distilled water to make a soft material, granulate with a 16-mesh sieve, dry in a drying oven at 40-45°C, add magnesium stearate to the dry granules Mix well and press into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com