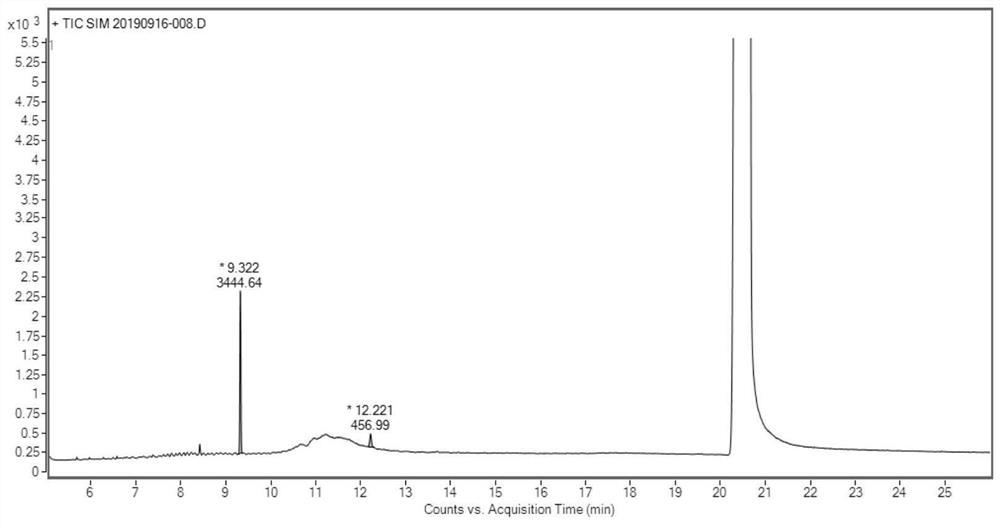
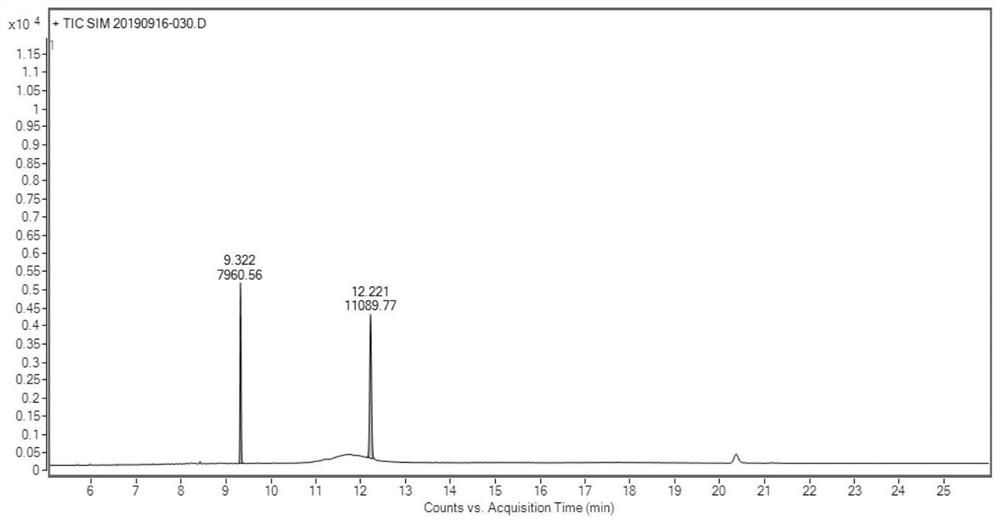
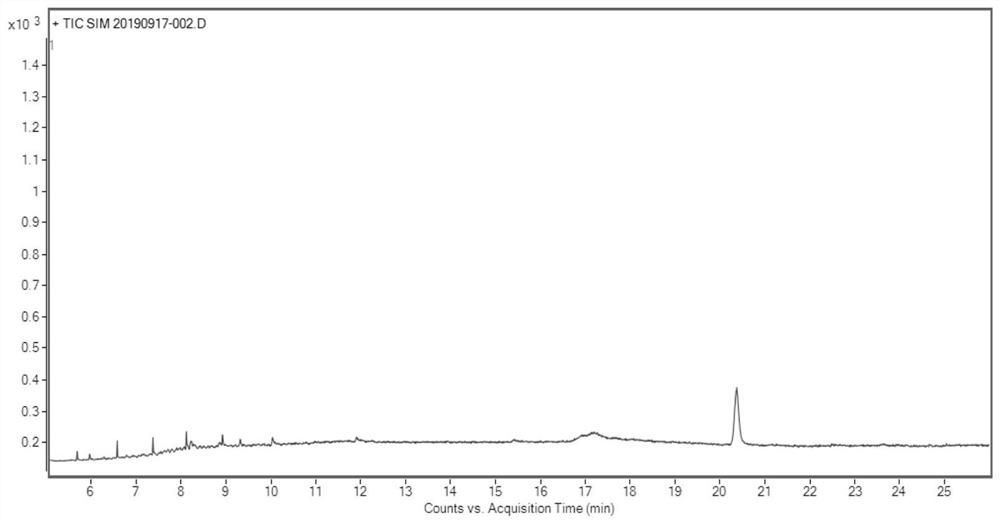
Determination method of oritavancin intermediate impurity 4-chlorobiphenyl and 4,4-dichlorobiphenyl content
A technology for oritavancin and determination method, which is applied to measurement devices, instruments, scientific instruments, etc., can solve the problems of interference detection, insufficient sensitivity, easy high temperature degradation of oritavancin intermediate side chains, etc., and achieves improved accuracy. , Improve sensitivity, good effect of impurity recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] 1. Detection
[0058] A. Preparation of the test solution:
[0059] Accurately weigh 0.05 g of the oritavancin side chain sample shown in formula I into a stoppered test tube, add 4 mL of acetonitrile, shake to dissolve the sample completely, then precisely add 5.0 mL of n-hexane, shake fully, and let stand Layering, take the upper n-hexane layer and set aside;
[0060] B. Preparation of reference substance solution:
[0061] Accurately weigh an appropriate amount of 4-chlorobiphenyl and 4,4-dichlorobiphenyl, mix and dissolve with N,N-dimethylformamide, quantitatively dilute to make a solution of 20 μg / mL each, and use it as the mother solution of the reference substance; Measure 0.01mL, 0.025mL, 0.04mL, 0.05mL and 0.06mL of the mother liquor of the reference substance, put them into a stoppered test tube, add 4mL of acetonitrile, shake and mix evenly, then precisely add 5.0mL of n-hexane, shake fully, and let stand to separate layer, take the upper n-hexane layer as...
Embodiment 2
[0069] A. Preparation of the test solution
[0070] Accurately weigh 0.1 g of the oritavancin side chain sample shown in formula I into a stoppered test tube, add 4 mL of acetonitrile, shake to dissolve the sample completely, then precisely add 5.0 mL of n-hexane, shake fully, and let stand Layering, take the upper n-hexane layer and set aside;
[0071] Steps B and C refer to Example 1.
[0072] According to the above conditions, three batches of test solution were detected, and the results are shown in Table 2.
[0073] Table 2:
[0074]
[0075]
Embodiment 3
[0077] A. Preparation of the test solution
[0078] Accurately weigh 0.05 g of the oritavancin side chain sample shown in formula I into a stoppered test tube, add 5 mL of acetonitrile, shake to dissolve the sample completely, then precisely add 5.0 mL of n-hexane, shake fully, and let stand Layering, take the upper n-hexane layer and set aside;
[0079] Steps B and C refer to Example 1.
[0080] According to the above conditions, three batches of test solution were detected, and the results are shown in Table 3.
[0081] table 3:
[0082]
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com