Purification method for recombinant human follicle-stimulating hormone
A technology of human follicle-stimulating hormone and purification method, applied in the fields of peptide preparation, chemical instruments and methods, organic chemistry, etc., can solve the problems of affecting protein biological activity, low activity recovery rate, unclear inactivation mechanism, etc. Avoid protein precipitation and sugar shedding problems, avoid recovery loss, and ensure the effect of purity and activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
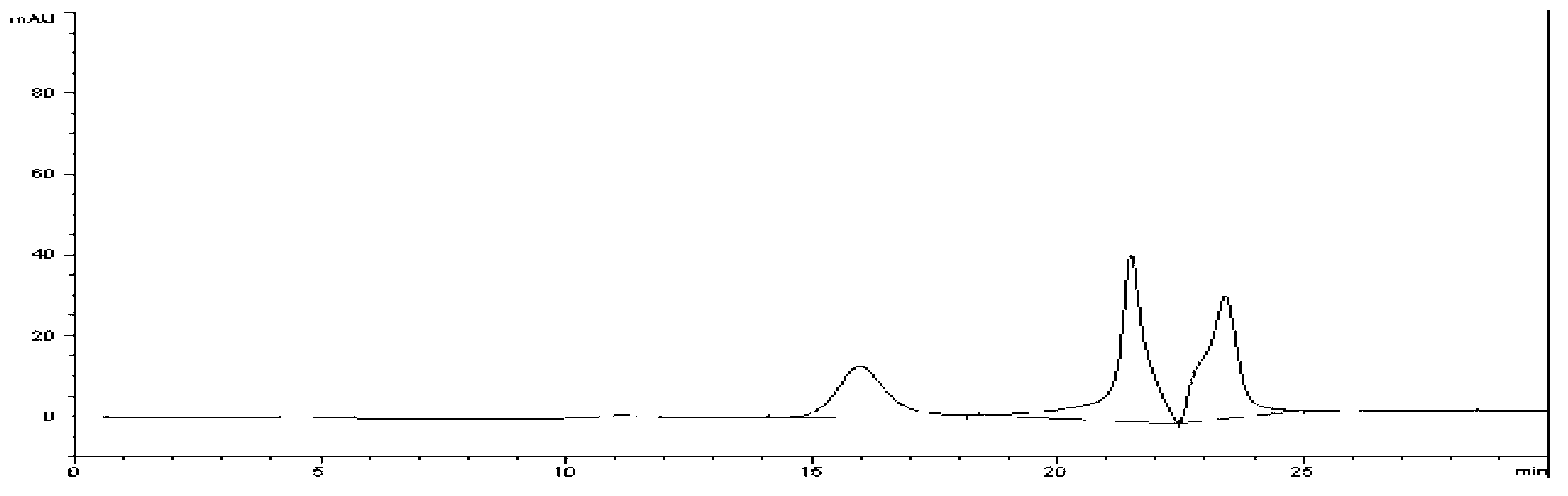
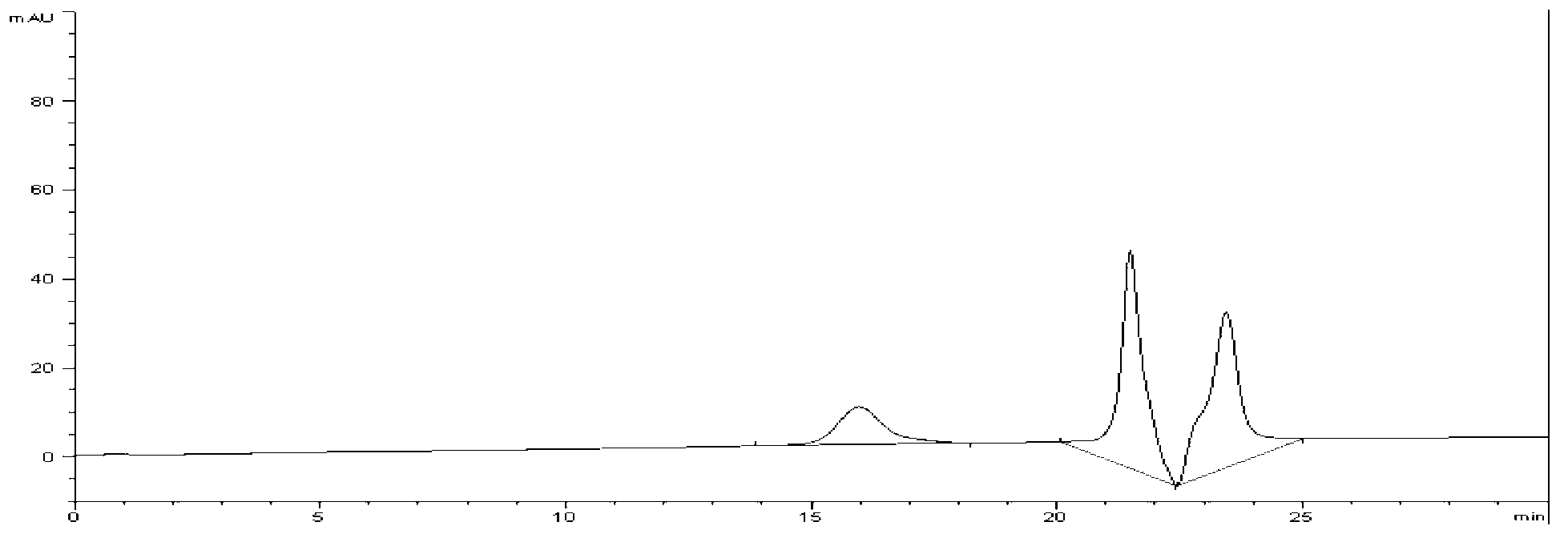
Image
Examples
Embodiment 1
[0040] Example 1 Purification Approach 1 of Recombinant Human Follicle-stimulating Hormone Protein
[0041] 1) Pretreatment of cell culture supernatant containing recombinant human FSH
[0042] Clean the ultrafiltration system and perform depyrogenation treatment, concentrate the supernatant, and replace the concentrate with (0.02MTris+0.2MNaCl+0.1M methionine) buffer (pH7.0).
[0043] 2) Q sepharose F.F anion exchange chromatography
[0044] To clean the Q sepharose F.F column chromatography system and perform depyrogenation treatment, follow the steps below:
[0045] Equilibrate the chromatographic column with phase A (0.02MTris+0.2MNaCl+0.1M methionine) buffer (pH7.0) and load the sample. Under this condition, the impurities are combined on the anion exchange column, while recombinant human follicle stimulating hormone The protein does not bind to the anion exchange column, and the flow-through fraction is collected.
[0046] 3) Affinity chromatography
[0047] Clean th...
Embodiment 2
[0051] Example 2 The Purification Approach Two of Recombinant Human Follicle Stimulating Hormone Protein
[0052] 1) Pretreatment of cell culture supernatant containing recombinant human FSH
[0053] Clean the ultrafiltration system and perform depyrogenation treatment, concentrate the supernatant, and replace the concentrate with (0.02MTris+0.1MNaCl+0.01Mmethionine) buffer (pH7.2).
[0054] 2) DEAE sepharose F.F anion exchange chromatography
[0055] To clean the DEAE sepharose F.F column chromatography system and perform depyrogenation treatment, follow the steps below:
[0056] Equilibrate the chromatographic column with phase A (0.02MTris+0.1MNaCl+0.01M methionine) buffer (pH7.2) and load the sample. Under this condition, the impurities are combined on the anion exchange column, while recombinant human follicle-stimulating hormone The protein does not bind to the anion exchange column, and the flow-through fraction is collected.
[0057] 3) Affinity chromatography
[0...
Embodiment 3
[0062] Example 3 The Purification Approach Three of Recombinant Human Follicle-stimulating Hormone Protein
[0063] 1) Pretreatment of cell culture supernatant containing recombinant human FSH
[0064] Clean the ultrafiltration system and perform depyrogenation treatment, concentrate the supernatant, and replace the concentrate with (0.05MTris+0.2MNaCl+0.1M methionine) buffer (pH7.5).
[0065] 2) Q sepharose F.F anion exchange chromatography
[0066] To clean the Q sepharose F.F column chromatography system and perform depyrogenation treatment, follow the steps below:
[0067] Equilibrate the chromatographic column with phase A (0.05MTris+0.2MNaCl+0.1M methionine) buffer (pH7.5) and load the sample. Under this condition, the impurities are combined on the anion exchange column, while recombinant human follicle stimulating hormone The protein does not bind to the anion exchange column, and the flow-through fraction is collected.
[0068] 3) Affinity chromatography
[0069] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com