Reverse-phase chromatography and mass-spectrometry combined detection method for complete low-molecular-heparin degradation product through precolumn derivatization
A low-molecular-weight heparin, completely degraded technology, used in measurement devices, material separation, analysis of materials, etc., can solve problems such as qualitative analysis and no reducing hemiacetal hydroxyl groups
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
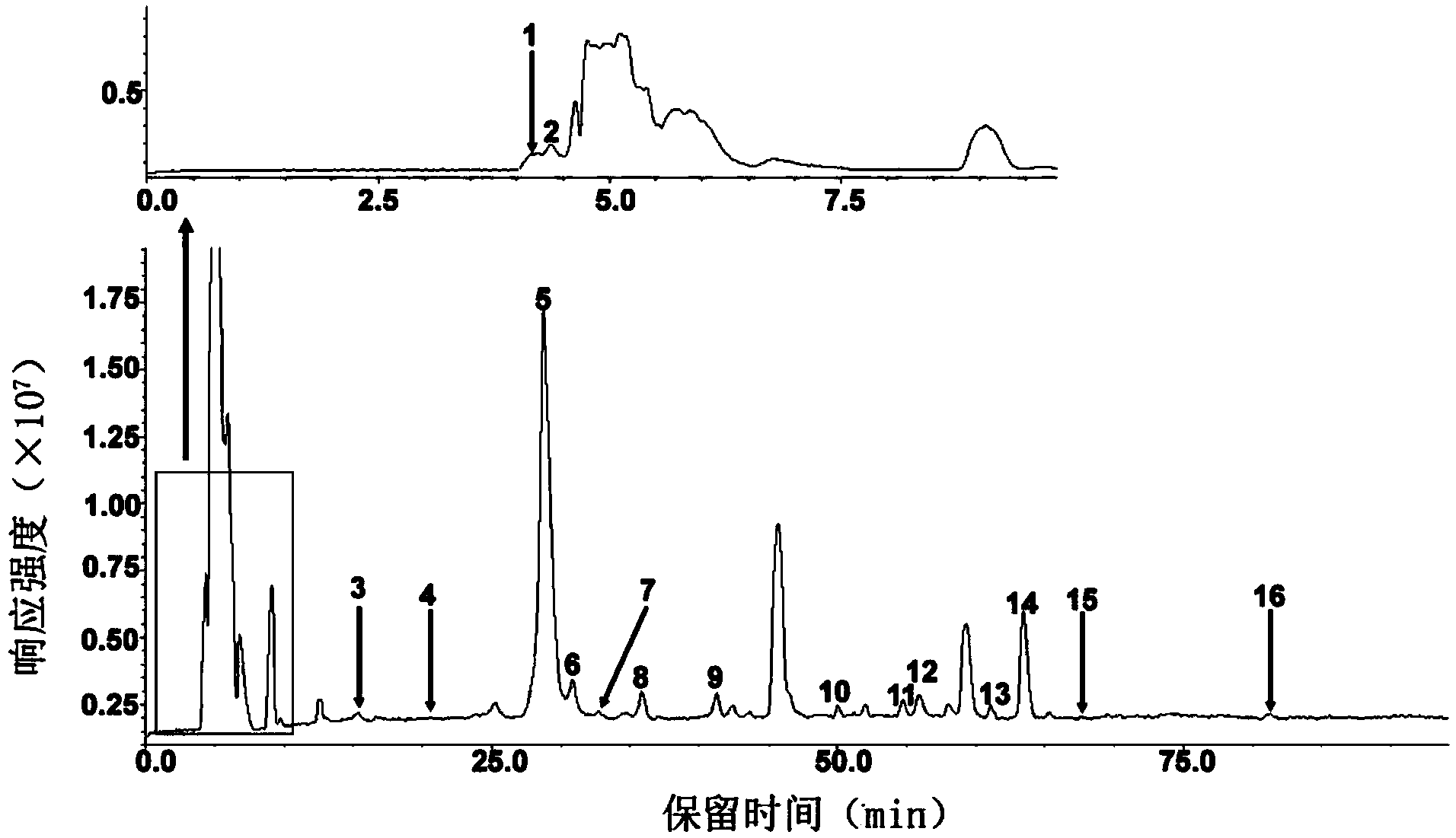
[0006] A reversed-phase chromatography-mass spectrometry detection method for complete degradation products of low-molecular-weight heparin combined with pre-column derivatization, the steps are as follows:
[0007] (1) Dissolve the ammonium acetate reagent in deionized water, and adjust the pH to 3-6 to prepare mobile phase A with an ammonium acetate concentration of 20-80 mM;
[0008] (2) methanol as mobile phase B;
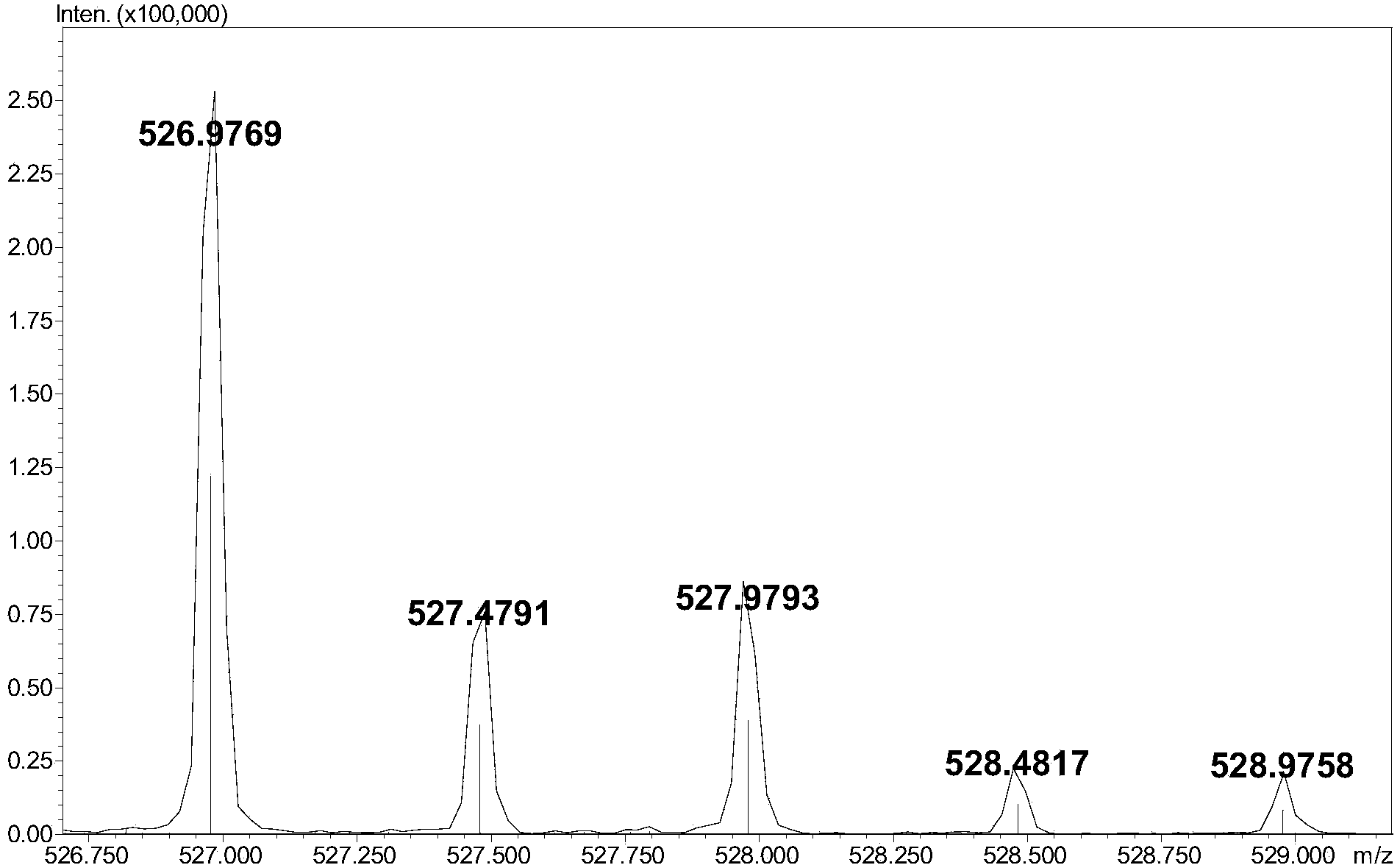
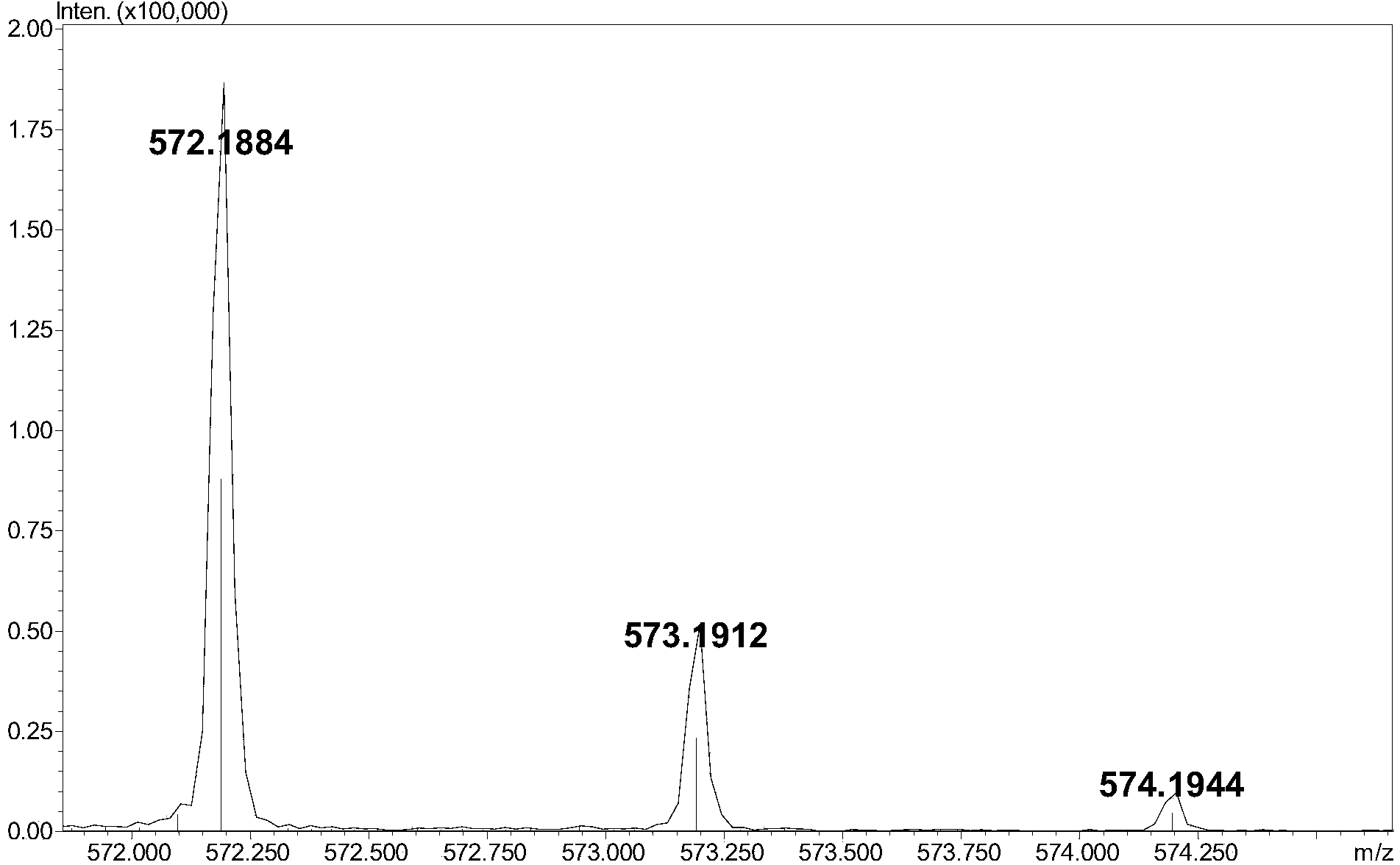
[0009] (3) Add 5-10 μL of 0.1M 2-aminoacridone (AMAC) solution to 10-50 μg of low-molecular-weight heparin complete enzymatic hydrolysis sample containing internal standard, let it stand at room temperature for 10-20 minutes, and then add 5-10 10 μL of 1 M sodium cyanoborohydride (NaBH 3 CN) solution, incubate at 45°C for 2-4 hours to obtain the derivatized sample;
[0010] (4) Dilute the derivatized sample prepared in step (3) with a 50% dimethyl sulfoxide (DMSO) solution to obtain a test solution with a derivatized sample concentration of 1-5 μg / μL. After ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com