Obeticholic acid crystal form I, preparation method, pharmaceutical composition, and application thereof
A technology of obeticholic acid and crystal form, which is applied in the field of medicinal chemical crystallization, can solve the problems of polluted environment and poor stability, and achieves the effects of simple steps, good stability and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
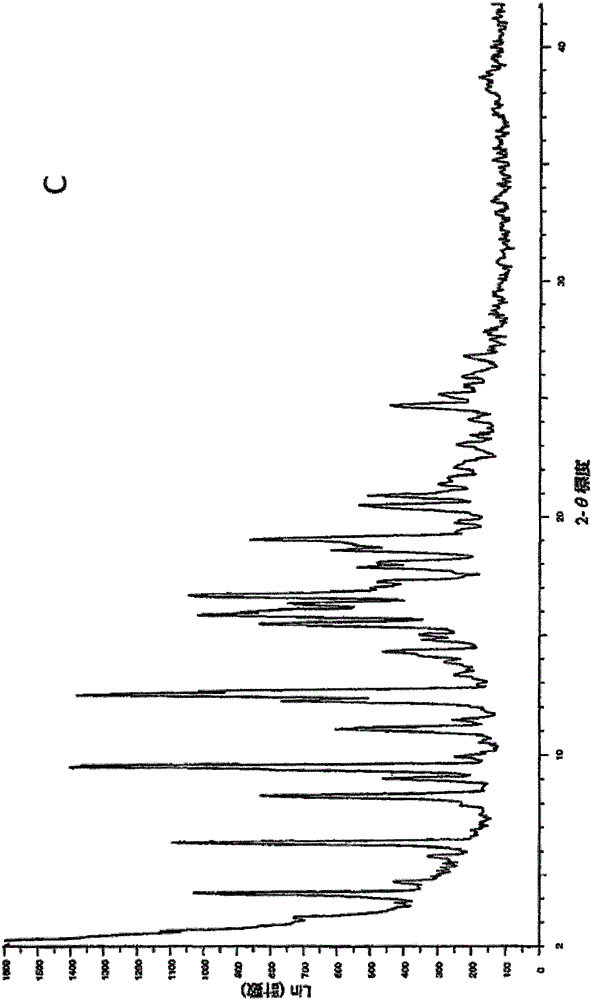
[0056] Obeticholic acid Form C was prepared according to Example 1, Reaction 6 of the patent document WO2013192097A1, and the specific operations were as follows:
[0057] 3α-Hydroxy-6α-ethyl-7-keto-5β-cholan-24-oic acid (86 g, 205.4 mmol), water (688 mL) and 50% (w / w) sodium hydroxide solution (56.4 mL) The mixture of was reacted with sodium borohydride (7.77 g, 205.4 mmol) in a mixture of 50% (w / w) sodium hydroxide solution (1.5 mL) and water (20 mL) at 90°C to 105°C. Heating to reflux under stirring for at least 3 hours, after the reaction, the reaction solution was cooled to 80°C. A mixture of citric acid (320.2 g, anhydrous), n-butyl acetate (860 mL) and water (491 mL) was added at 30° C. to 50° C. to ensure an acidic pH, and the aqueous phase was separated. The organic phase was distilled, and the distilled residue was diluted with n-butyl acetate, cooled slowly to 15°C to 20°C, and centrifugally filtered. The crude product was crystallized from n-butyl acetate. Obeti...
Embodiment 1
[0060] Take 50 mg of obeticholic acid form C, add 1 mL of n-butyl acetate to dissolve, and evaporate to dryness at 50°C to obtain obeticholic acid crystal form I.
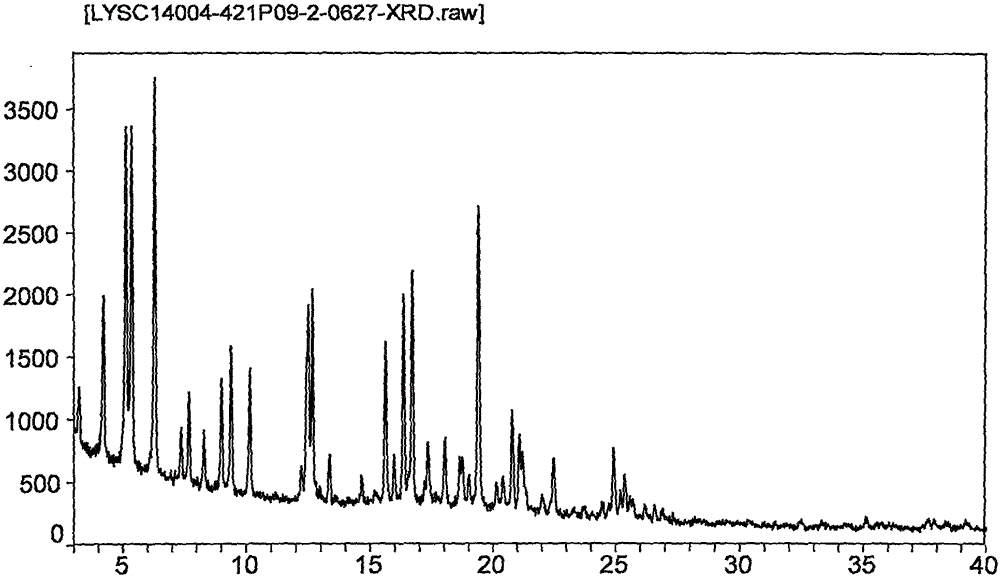
[0061] The XRPD pattern of crystal form I is as follows figure 2 shown. Appears as crystalline material.
[0062] The DSC spectrum of crystal form I is as follows image 3 shown. It shows a melting point of 77°C.
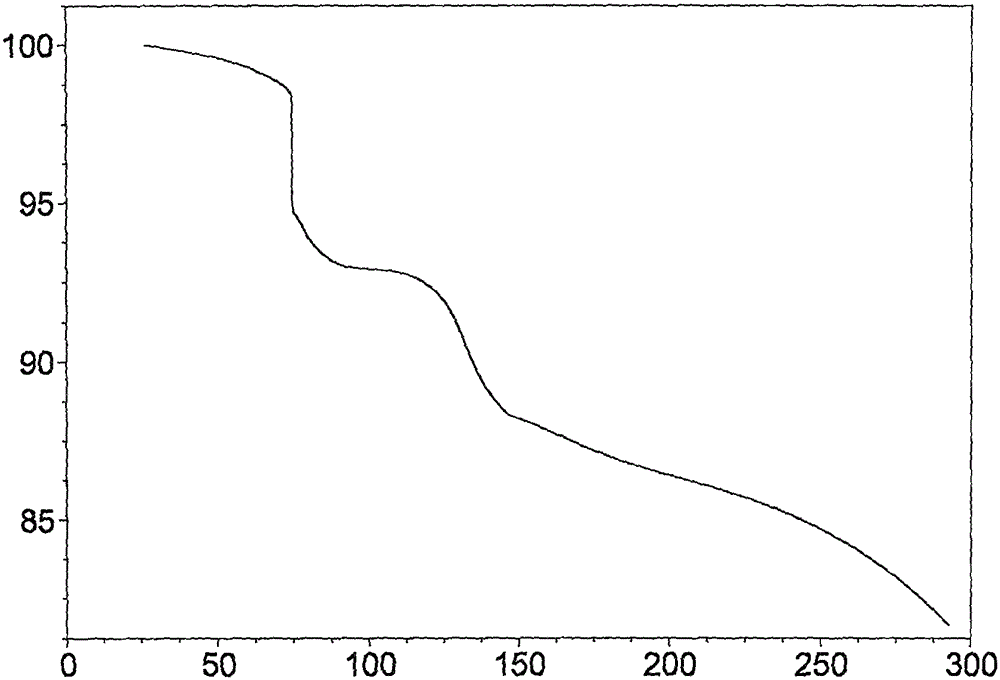
[0063] The TGA spectrum of crystal form I is as follows Figure 4 shown. It showed 7.07% weight loss before 100°C and 11.79% weight loss before 150°C.
[0064] The DVS spectrum and isotherm adsorption curve of crystal form I are as follows: Figure 5 and Image 6 shown. It shows that the weight change is 0.57% in the range of 20%-80% relative humidity, and the weight change is 0.69% in the whole process.
[0065] The infrared spectrum of crystal form I is as follows Figure 7 shown. Display: at wave numbers of 2930, 1707, 1450, 1364, 1262, 1242, 1161, 1121, 1062, 1045, 973, 954 and 808cm -1 ...
Embodiment 2
[0067] Take 50 mg of obeticholic acid form C, add 0.25 mL of n-butyl acetate to dissolve, and volatilize at room temperature to obtain obeticholic acid crystal form I single crystal.
[0068] The single crystal unit cell parameters are shown in Table 1.
[0069] Table 1 Single Crystal Unit Cell Parameters of Form I
[0070]
[0071]
[0072] In Table 1, a, b, c represent the unit cell axis length, α, β, γ represent the dihedral angle, Z represents the number of molecules in each unit cell, V represents the unit cell volume, D calc represents the unit cell density.
[0073] Single crystal analysis related parameters: residual factor R1=0.0568, weighted R value wR2=0.1453, goodness of fit GooF(S)=0.917. The R1 value is less than 0.06, the wR2 value is less than 0.15, and the S value is close to 1, indicating that the single crystal data is reasonable.
[0074] The results proved that the crystal form I was composed of obeticholic acid molecules and was an anhydrous subs...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com