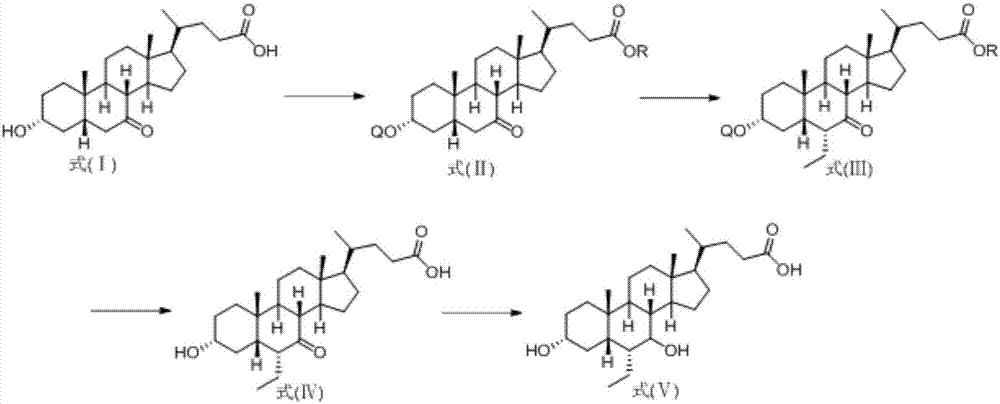
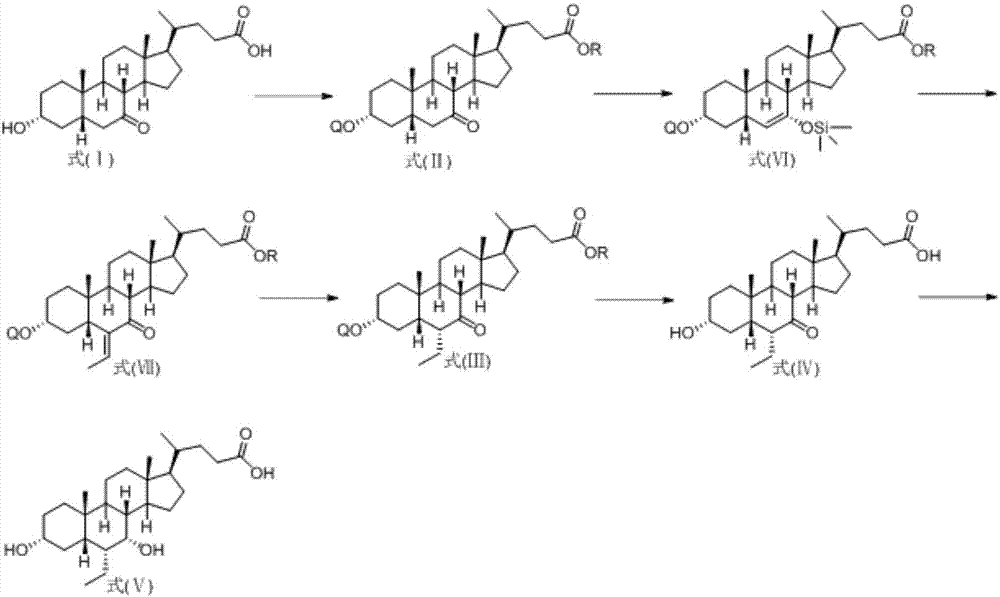
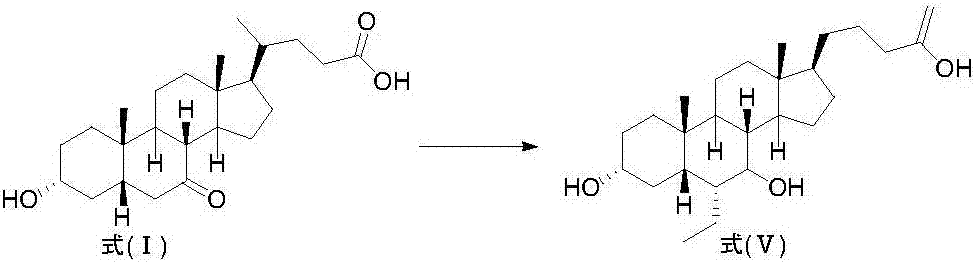
Method for preparing obeticholic acid from new derivative of 3alpha-hydroxy-7-oxo-5beta-cholanic acid
A technology of obeticholic acid and cholanic acid, which is applied in the field of medicine, can solve the problems of large 7-position chiral inversion impurities, difficult purification and separation of oily substances, etc., and achieves easy purification, significant creativity and practical application value, The effect of increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] a) Preparation of 3α-hydroxyl-7-oxo-5β-cholanic acid methyl ester
[0021] 3α-Hydroxy-7-oxo-5β-cholanic acid (39.0 g, 100 mmol) and p-toluenesulfonic acid (1.72 g, 10 mmol) were dissolved in 120 ml of methanol, heated to reflux, and kept for 1 hour. Cool down to room temperature, and slowly add 240ml of water to dilute with stirring, and a solid precipitates out. After the dropwise addition, cool to 5°C, stir for 1 hour, and filter. The solid was rinsed and dried with a methanol:water (1:10) solution to obtain methyl 3α-hydroxy-7-oxo-5β-cholanate (37.6 g, yield 93.1%).
[0022] b) Preparation of 3α-triphenylmethoxy-7-oxo-5β-cholanoic acid methyl ester
[0023] 3α-Hydroxy-7-oxo-5β-cholanoic acid methyl ester (40.4g, 100mol), triphenylchloromethane (29.2g, 105mmol), DMAP (4-dimethylaminopyridine) (1g) were dissolved in In 120ml of DMF (N,N-dimethylformamide), the temperature was raised to 50°C with stirring, and the reaction was kept for 24 hours. Cool down to 20°C, a...
Embodiment 2
[0034] a) Preparation of ethyl 3α-hydroxy-7-oxo-5β-cholanate
[0035] 3α-Hydroxy-7-oxo-5β-cholanic acid (39.0 g, 100 mmol) and p-toluenesulfonic acid (1.72 g, 10 mmol) were dissolved in 120 ml of ethanol, heated to reflux, and kept for 1 hour. Cool down to room temperature, and slowly add 240ml of water to dilute with stirring, and a solid precipitates out. After the dropwise addition, cool to 5°C, stir for 1 hour, and filter. The solid was rinsed and dried with ethanol alcohol:water (1:10) solution to obtain ethyl 3α-hydroxy-7-oxo-5β-cholanate (39.8 g, yield 93.1%).
[0036] b) Preparation of 3α-p-benzyloxymethoxy-7-oxo-5β-cholanoic acid ethyl ester
[0037] 3α-Hydroxy-7-oxo-5β-cholanoic acid ethyl ester (41.8g, 100mmol), p-benzyloxychloromethyl ether (17.2g, 110mmol), tetrabutylammonium iodide (1g) were dissolved in 150ml In dichloromethane, diisopropylethylamine (110 mmol) was slowly added dropwise with stirring. After the dropwise addition, the reaction was carried out...
Embodiment 3
[0050] a) Preparation of tert-butyl 3α-hydroxy-7-oxo-5β-cholanate
[0051] Dissolve 3α-hydroxy-7-oxo-5β-cholanic acid (39.0g, 100mmol) and tert-butanol (8.9g, 120mmol) in 180ml of dichloromethane, cool to 5°C in an ice-water bath, add sulfuric acid: sulfuric acid Magnesium (1:4) 5g, stirred for 1 hour, naturally warmed to room temperature, and reacted for 6 hours. Add 100ml of water to wash, separate the organic phase, wash with saturated brine 50ml*2, dry with anhydrous sodium sulfate, filter, and concentrate under reduced pressure to obtain tert-butyl 3α-hydroxy-7-oxo-5β-cholanate (42.9 g, yield 86.1%).
[0052] b) Preparation of tert-butyl 3α-benzyloxy-7-oxo-5β-cholanate
[0053] 3α-Hydroxy-7-oxo-5β-cholanoic acid tert-butyl ester (44.6g, 100mmol), triethylamine (12.5g, 120mmol) were dissolved in 200ml tetrahydrofuran, and benzyl bromide (18.0 g, 105mmol), the dropwise addition was completed and incubated for 4 hours. Add 100 ml of saturated brine for washing, separate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com