Obeticholic acid compound, and medicinal composition containing compound
A technology of obeticholic acid and its compounds, which is applied in the field of obeticholic acid compounds and their pharmaceutical compositions and preparations, can solve problems affecting the quality of medicines, and achieve good reproducibility and stable preparation processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Preparation of crystal form of obeticholic acid
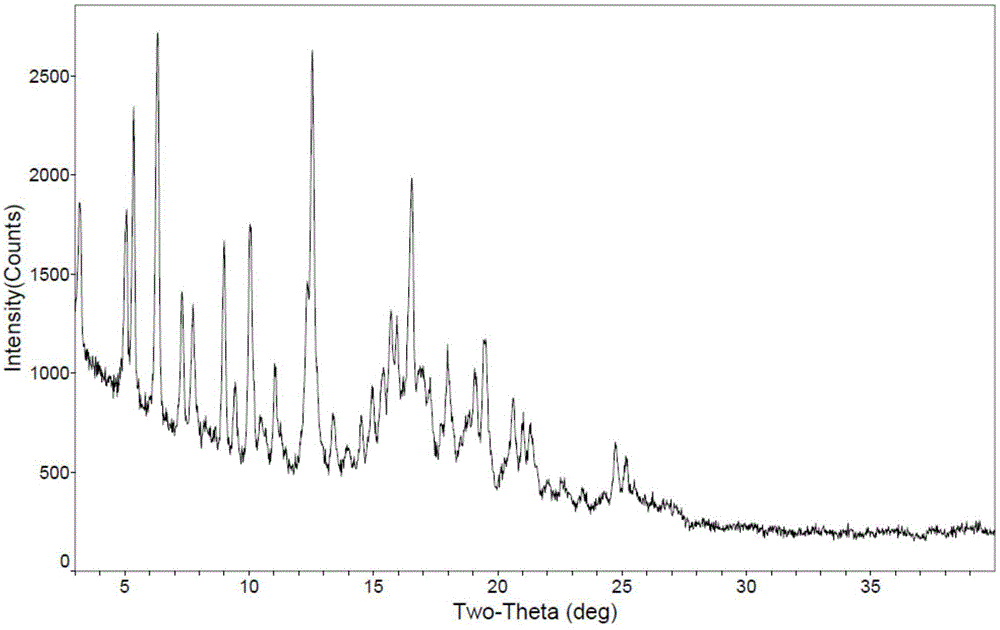
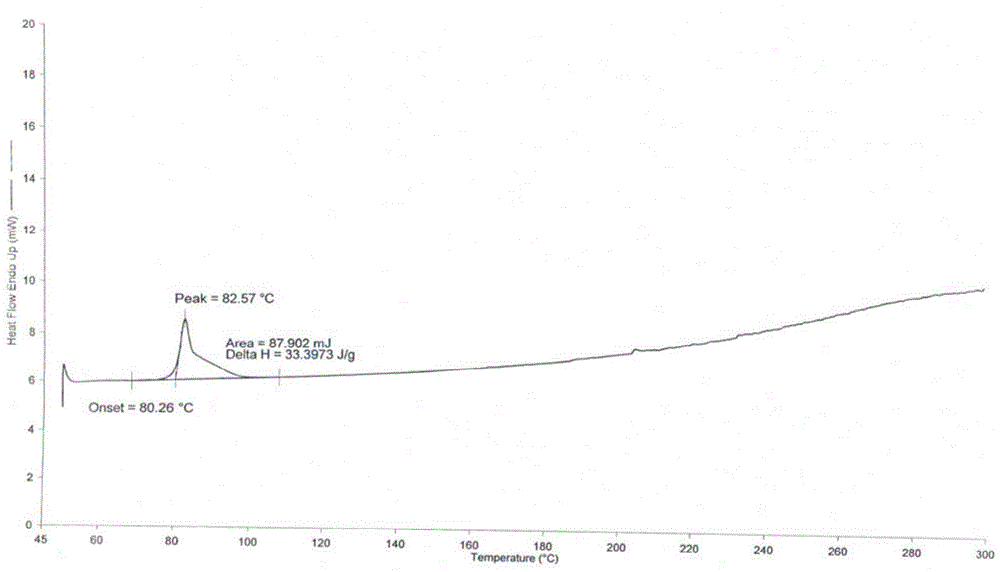
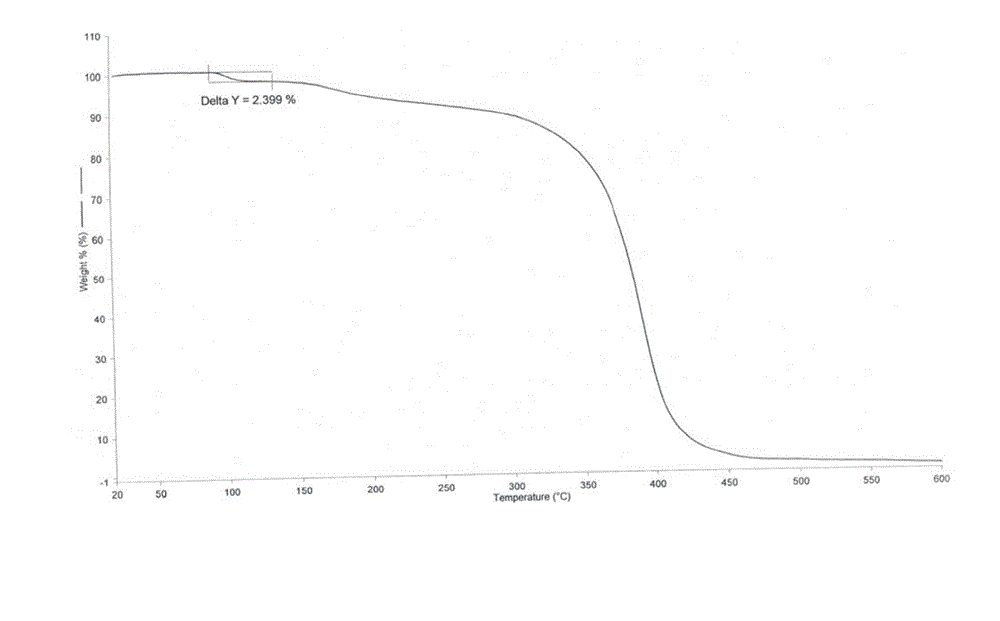
[0042] 3α,7α-dihydroxy-6α-ethyl-5β-cholan-24-acid methyl ester (65.9g, 0.152mol), methanol (152ml), 20% (mass fraction) sodium hydroxide aqueous solution (36ml) Mix and react at 55~60℃ for 2h. After the reaction was completed, methanol was evaporated under reduced pressure, the residue was dissolved in 150 ml of purified water, and concentrated hydrochloric acid was added dropwise with stirring to adjust the pH to 2-3. Ethyl acetate (200ml) was added to the solution, and the solution was extracted and separated. The organic phase was dried with anhydrous sodium sulfate for 2h and then spin-dried to obtain about 60.6g of yellow oil. Then n-butyl acetate (73ml) was added to dissolve it at 60°C. , after dropping to room temperature, a white solid was precipitated, filtered, and the filter cake was dried at 40° C. to obtain 41.9 g of obeticholic acid solid. The X-ray powder diffraction (XRD) pattern, differential...
Embodiment 2
[0043] Example 2 Preparation of crystal form of obeticholic acid
[0044]Add obeticholic acid (82g) to the reaction flask, add ethyl acetate (100ml) and heat to 60°C to dissolve all, stand and cool down to room temperature naturally, separate out white solids, filter, and dry the filter cake at 40°C to obtain 60g of solids . X-ray powder diffraction (XRD) pattern attached figure 1 basically the same.
Embodiment 3
[0045] Example 3 Preparation of crystal form of obeticholic acid
[0046] Add obeticholic acid (98.0g) to the reaction flask, add ethyl acetate (195ml) and heat to 60°C to dissolve, let stand and drop to room temperature to precipitate a white solid, filter, and dry the filter cake at 40°C to obtain Product 66.6 g. X-ray powder diffraction (XRD) pattern attached figure 1 basically the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com