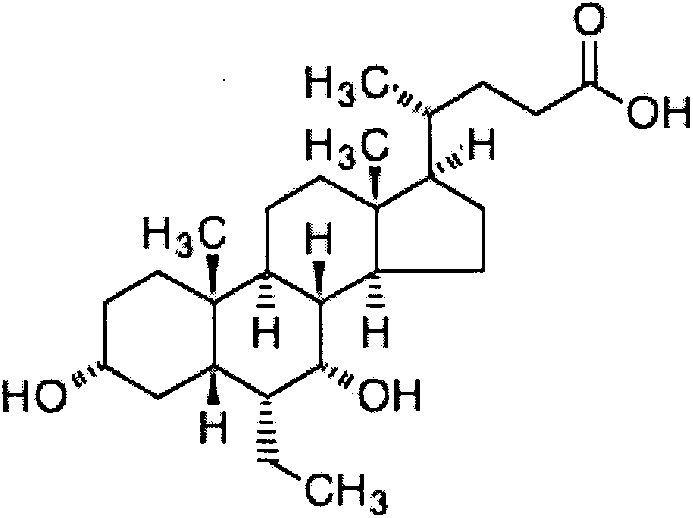
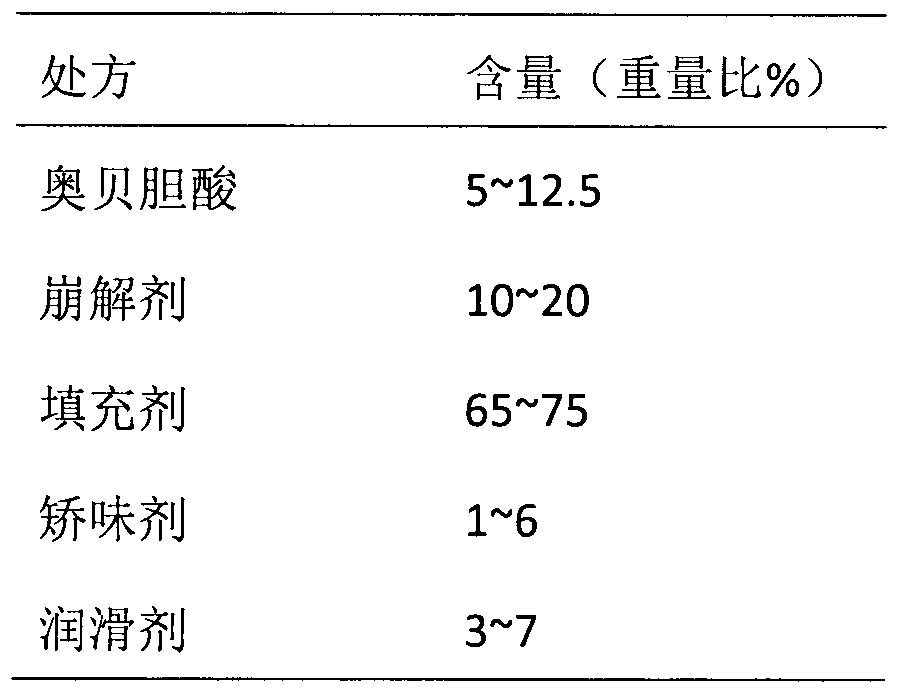
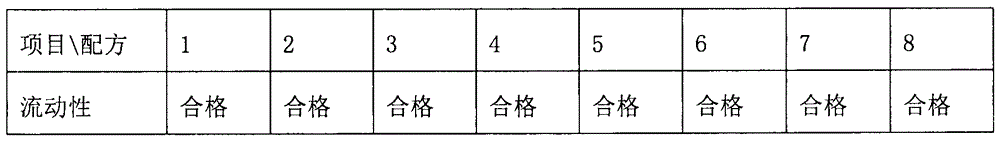
Oral disintegrating tablet of obeticholic acid, and preparation method thereof
A technology of obeticholic acid and orally disintegrating tablets, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., can solve complex equipment, high production costs, fluidity of excipients, Issues such as large impact on compressibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] To produce 1000 tablets of obeticholic acid orally disintegrating tablets with a specification of 10 mg, the dosage of raw and auxiliary materials is as follows:
[0058] Raw materials
Dosage (g)
10
Calcium Carboxymethyl Cellulose
20
120
30
12
8
[0059] Preparation:
[0060] (a) The main drug obeticholic acid is pulverized through a 100-mesh sieve, and the filler is passed through a 100-mesh sieve for subsequent use;
[0061] (b) The main ingredient is mixed with the pregelatinized starch, sieved, and the filler sorbitol is added and mixed;
[0062] (c) adding disintegrant, flavoring agent and lubricant to the mixture obtained in step (b) above and mixing;
[0063] (d) Tablets.
Embodiment 2
[0065] To produce 1000 tablets of obeticholic acid orally disintegrating tablets with a specification of 10 mg, the dosage of raw and auxiliary materials is as follows:
[0066] Raw materials
[0067] The preparation method is the same as in Example 1.
Embodiment 3
[0069] To produce 1000 tablets of obeticholic acid orally disintegrating tablets with a specification of 25 mg, the dosage of raw and auxiliary materials is as follows:
[0070] Raw materials
[0071] The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com