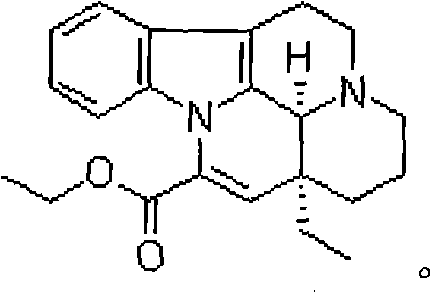
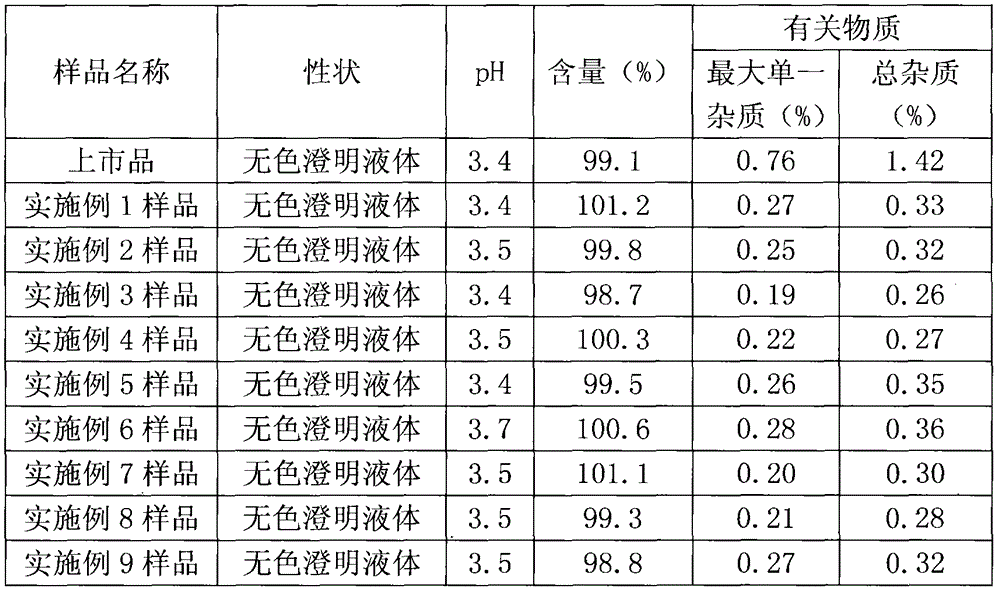
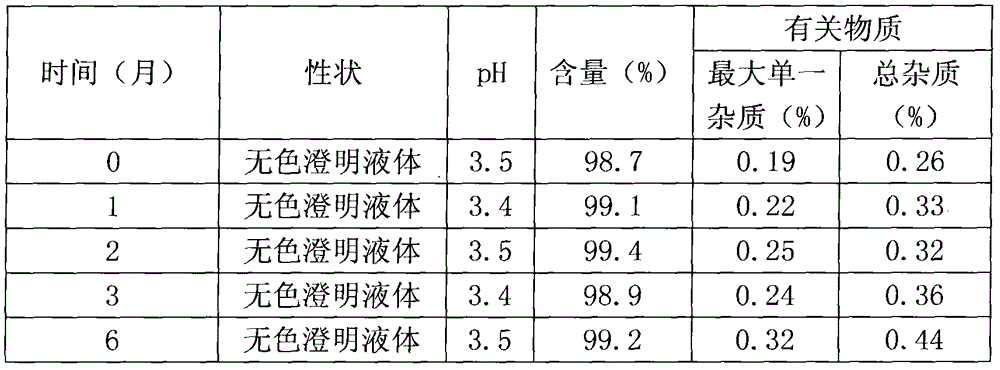
Medical composition
A technology of vinpocetine and citric acid, which is applied in the field of liquid preparations containing vinpocetine and its preparation, and can solve the problems of vinpocetine injection research reports that have not been seen, the production process is sterile, the water quality is low, and high temperature is not included Sterilization steps and other issues, to achieve the effect of simplifying the composition of the prescription, improving stability, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The prescription is as follows:
[0032] Vinpocetine 10g
[0033] Citric acid 5g
[0034] Water was added to 2000ml.
[0035] Preparation method: add citric acid into 80% water and stir to dissolve completely, add 1mol / l phosphoric acid to adjust pH to 2.2, add vinpocetine, stir to dissolve completely, add 0.1mol / l NaOH Adjust the pH of the aqueous solution to 3.4, add water to a sufficient amount, add injection-grade activated carbon (0.1%, w / v), insulate and stir at 60°C for 30 minutes, filter, fill, 2ml each, and sterilize at 121°C for 15 minutes. That is, Vinpocetine Injection.
Embodiment 2
[0037] The prescription is as follows:
[0038] Vinpocetine 10g
[0039] Citric acid 8g
[0040] Water was added to 2000ml.
[0041] Preparation method: add citric acid to 80% water and stir to dissolve it completely, add 1mol / l hydrochloric acid to adjust the pH to 2.0, add vinpocetine, stir to dissolve it completely, add 0.1mol / l NaOH Adjust the pH of the aqueous solution to 3.4, add water to a sufficient amount, add injection-grade activated carbon (0.1%, w / v), insulate and stir at 60°C for 30 minutes, filter, fill, 2ml each, and sterilize at 121°C for 15 minutes. That is, Vinpocetine Injection.
Embodiment 3
[0043] The prescription is as follows:
[0044] Vinpocetine 10g
[0045] Citric acid 10g
[0046] Water was added to 2000ml.
[0047] Preparation method: add citric acid to 80% water and stir to dissolve it completely, add vinpocetine, stir to dissolve it completely, add 0.1mol / l NaOH aqueous solution to adjust the pH to 3.4, add water to a sufficient amount , add injection-grade activated carbon (0.1%, w / v) at 60 DEG C and insulate and stir for adsorption for 30 minutes, filter, fill, each of 2ml, and sterilize at 121 DEG C for 12 minutes to obtain Vinpocetine Injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com