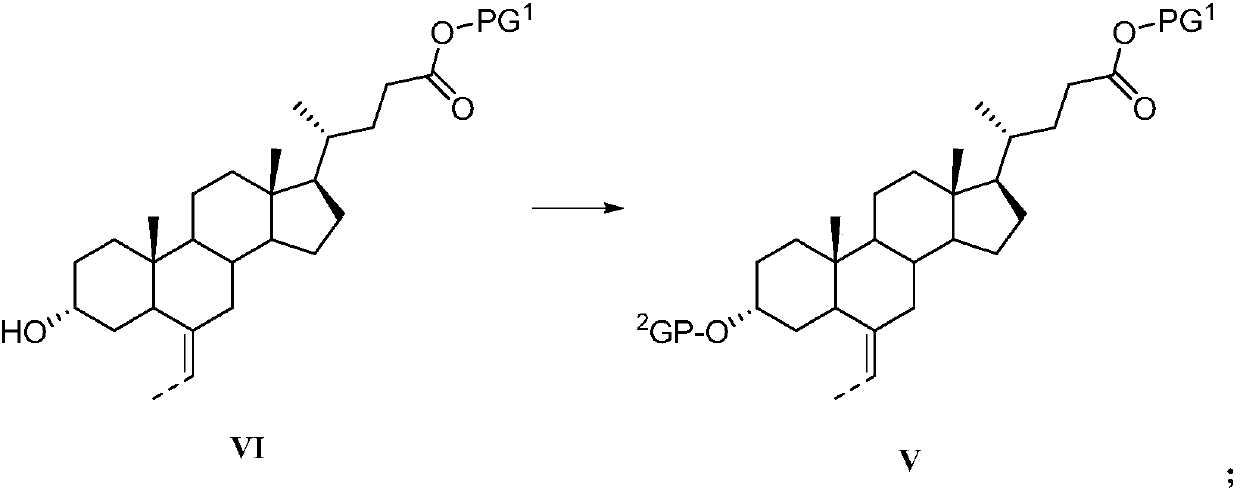
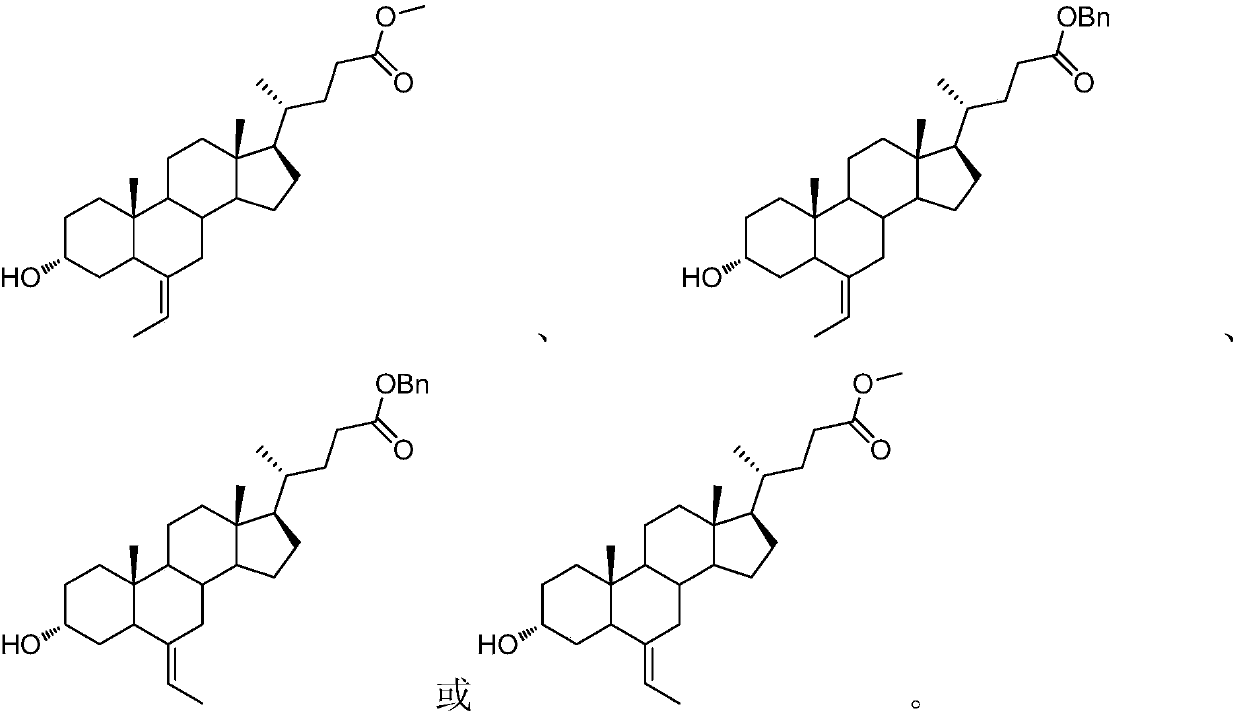
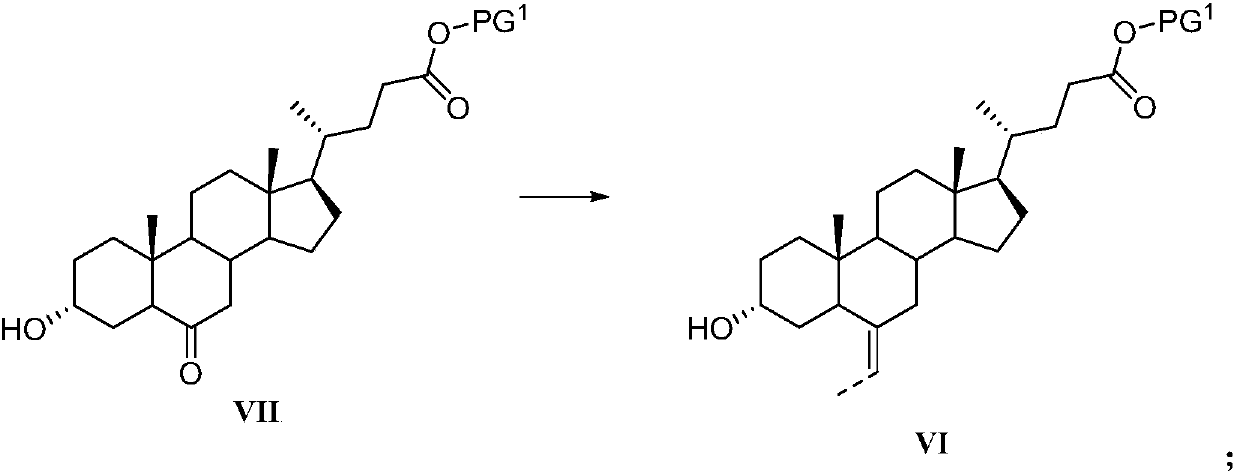
Preparation method of obeticholic acid and intermediate thereof
A volume and compound technology, applied in the production of steroids, organic chemistry, bulk chemicals, etc., can solve the problems of large pollution, complicated operation and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0231] The preparation of embodiment 1 compound 1
[0232]
Embodiment 2
[0234] The preparation of embodiment 2 compound 1
[0235] N 2 Put ethyltriphenylphosphine bromide (242.4g, 653mmol), tetrahydrofuran (300ml), potassium tert-butoxide (111g, 990mmol) into a four-necked flask under protection, stir at 65°C for 30min, and add compound 19 (80g, 198mmol) in tetrahydrofuran solution (100ml), stirred for 1-2h, the reaction solution was acidified with 6mol / L hydrochloric acid, the solution was evaporated to dryness, ethyl acetate (500ml) and water (600ml) were added to extract and separate the liquid, and the organic layer was washed with saturated It was washed twice with 400 ml of saline, dried and evaporated to dryness, and a solid (58.8 g, 72.2%) was obtained by column chromatography. Identification data is the same as in Example 1.
Embodiment 3
[0236] The preparation of embodiment 3 compound 1
[0237] N 2 Put ethyltriphenylphosphine bromide (2.4g, 6.5mmol), tetrahydrofuran (10ml), potassium tert-butoxide (1.1g, 9.9mmol) into a four-necked flask under protection, stir at -10°C for 30min, and add the compound dropwise 19 (0.8g, 1.9mmol) in tetrahydrofuran (5ml), stirred for 1-2h, the reaction solution was acidified with 6mol / L hydrochloric acid, the solution was evaporated to dryness, and ethyl acetate (20ml) and water (20ml) were added to extract liquid, the organic layer was washed twice with 20 ml of saturated brine, dried and evaporated to dryness, and a solid (0.56 g, 68.4%) was obtained by column chromatography. Identification data is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com