Obeticholic acid compound and pharmaceutical composition thereof
A technology of obeticholic acid and its compounds, which is applied in the field of obeticholic acid compounds and their pharmaceutical compositions and preparations, can solve problems affecting the quality of medicines, and achieve good reproducibility and stable preparation processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1 Preparation of obeticholic acid crystal form
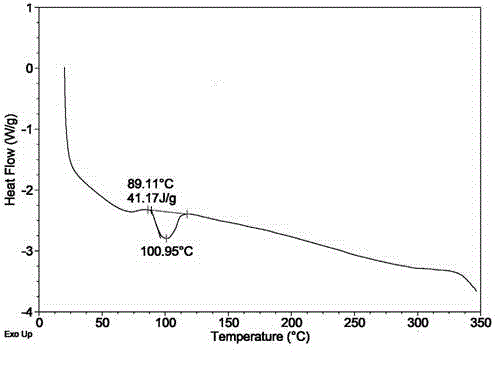
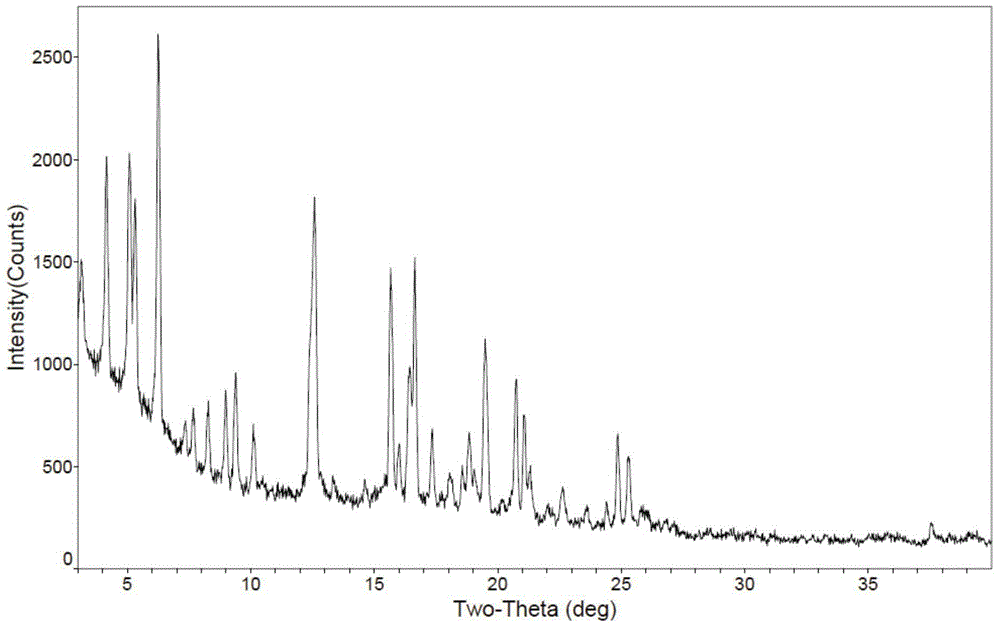
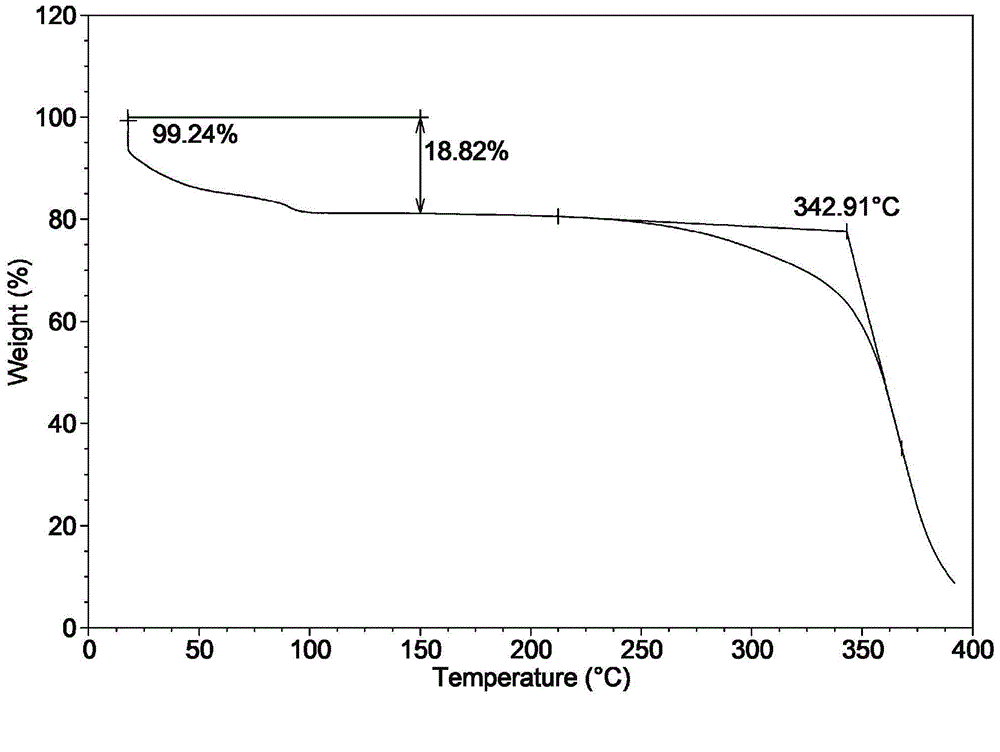
[0041] Dissolve 5g of obeticholic acid sample in a mixed solvent of chloroform / n-heptane (100mL / 150mL) in a water bath at 60°C, keep it for 30 minutes, then cool it naturally in a water bath to room temperature, precipitate a flocculent solid, filter and dry to obtain a new crystal type. X-ray powder diffraction (XRD) collection of illustrative plates, differential scanning calorimetry (DSC) collection of illustrative plates, thermogravimetric analysis (TGA) collection of illustrative plates are respectively as attached figure 1 , 2 , 3 shown.
Embodiment 2
[0042] Embodiment 2 Preparation of obeticholic acid crystal form
[0043] In a water bath at 60°C, 15g of obeticholic acid sample was dissolved in 300mL of chloroform, and was added dropwise into 500mL of n-heptane solvent under stirring to precipitate a flocculent solid, which was filtered and dried to obtain a new crystal form. X-ray powder diffraction (XRD) pattern attached figure 1 basically the same.
Embodiment 3
[0044] Embodiment 3 Preparation of obeticholic acid crystal form
[0045]At room temperature, 15g of obeticholic acid sample was dissolved in 300mL of dichloromethane, and was added dropwise into 500mL of n-hexane solvent under stirring to precipitate a flocculent solid, which was filtered and dried to obtain a new crystal form. X-ray powder diffraction (XRD) pattern attached figure 1 basically the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com