Method for preparing obeticholic acid intermediate
A technology for obeticholic acid and intermediates, which is applied in the field of preparing obeticholic acid intermediates, can solve problems such as unfavorable industrialized production, long process route, complicated operation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
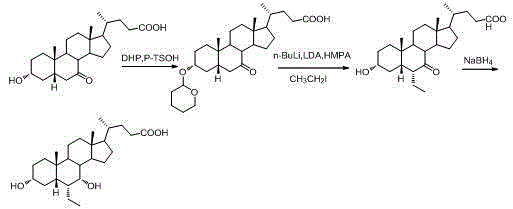
[0057] 1) Preparation of 3-α-hydroxy-6-(1-hydroxy)-ethyl-7-keto-5-β-cholanic acid
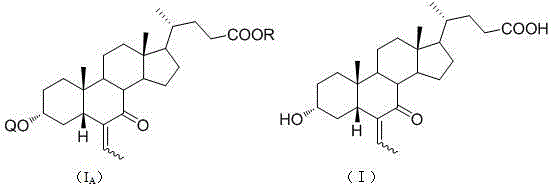
[0058] Dissolve 2g of 7-oxo-lithocholic acid (II) in 100ml of tetrahydrofuran, add 14.2ml of lithium diisopropylamide (1.8M) at -78°C, control the reaction at -78°C for 3h, and then add 3ml of ethyl alcohol Aldehyde, react at -78°C for 5 hours, add saturated ammonium chloride solution to quench the reaction, evaporate the reaction solvent tetrahydrofuran, then extract with ethyl acetate, evaporate to dryness to obtain oil, oil: methanol: water = 1:3:1 The product was refined to obtain 1.1g of the product with a yield of 49.42%. The product was detected by MS [M-1]=433.
[0059] 2) Preparation of 3-α-hydroxy-6-ethylidene-7-keto-5-β-cholanic acid
[0060] Dissolve 0.6g of 3-α-6-(1-hydroxy)-ethyl-7-keto-5-β-cholanic acid in 10ml of toluene, add 0.26g of p-toluenesulfonic acid monohydrate, and control the temperature React at about 60°C for 2 hours, then wash with saturated sodium bicarbonate unt...
Embodiment 2
[0062] 1) Preparation of 3-α-tetrahydropyranyl ether-7-keto-5β-cholanic acid
[0063] Dissolve 1Kg of 7-oxo-lithocholic acid (II) in a reaction kettle with 20L of dichloromethane, add 122g of p-toluenesulfonic acid monohydrate and 538g of dihydropyran in sequence, react at room temperature for 1 hour, add saturated carbonic acid Sodium hydrogen solution until the pH of the system is about 7, separate the liquids, extract the water layer with dichloromethane three times, combine the organic layers, dry to obtain a semi-solid, and refine with ethyl acetate:n-hexane=1:5 to obtain 1Kg of the product. Yield 82.3%
[0064] 2) Preparation of 3-α-tetrahydropyranyl ether-6-(1-hydroxy)-ethyl-7-keto-5-β-cholanic acid
[0065] Dissolve 1Kg of 3-α-tetrahydropyranyl ether-7-keto-5β-cholanic acid in 30L tetrahydrofuran, add 3.5L lithium diisopropylamide (1.8M) at -78°C, and keep it at low temperature Reacted for 3.5h, then added 465g of acetaldehyde. React at -78°C for 6 hours, add satura...
Embodiment 3
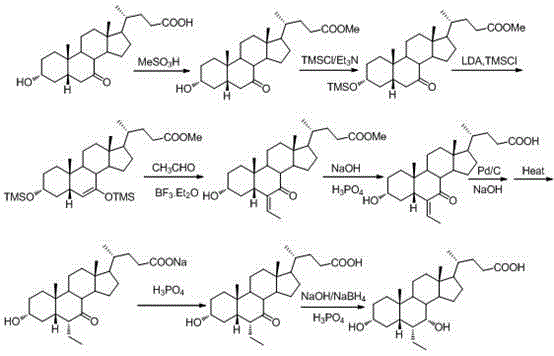
[0069] 1) Preparation of 3-α-acetoxy-7-keto-5-β-cholanic acid
[0070] Dissolve 2g of 7-oxo-lithocholic acid (II) in 10ml of acetonitrile, add 0.008g of dimethylaminopyridine and 1.05g of acetic anhydride, react under reflux for 4h, add water to obtain a solid, refine with acetonitrile to obtain 2.0g of the product, and collect The rate is 90.3%.
[0071] 2) Preparation of 3-α-acetoxy-6-(1-hydroxy)-ethyl-7-keto-5-β-cholanic acid
[0072] Dissolve 2g of 3-α-acetoxy-7-keto-5-β-cholanic acid in 150ml of tetrahydrofuran, add 13ml of lithium diisopropylamide (1.8M) at -78°C, and keep the reaction at low temperature for 4.5h , then add acetaldehyde 1.3ml, continue the reaction for 5h, quench the reaction with 10% hydrochloric acid, evaporate part of tetrahydrofuran, extract and separate liquid with ethyl acetate, evaporate to dryness to obtain 2.5g of oil. Oily matter: ethyl acetate: n-hexane = 1:10:5 and refined to obtain 1.3 g of the product, with a yield of 59.0%. The product w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com