Application of combination of Farnesoid XReceptor (FXR) agonist and apoptotic inhibitor in preparation of superior anti-hepatic fibrosis drugs
An apoptosis inhibitor and anti-hepatic fibrosis technology, applied in the field of medicine, can solve the problems of reduced drug efficacy, increased cholesterol level, decreased high-density lipoprotein level, etc., and achieves the effect of avoiding side effects and excellent anti-fibrosis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
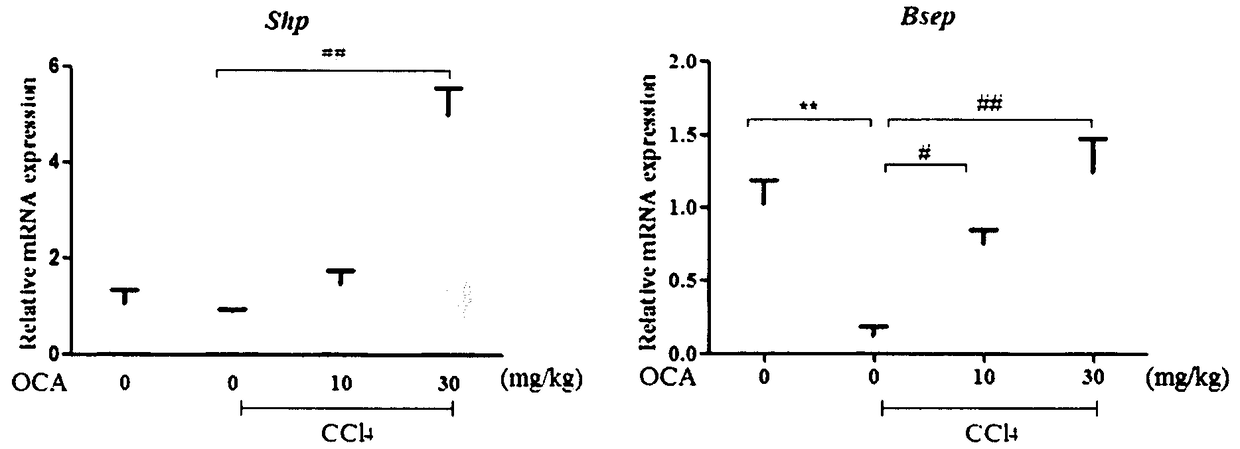
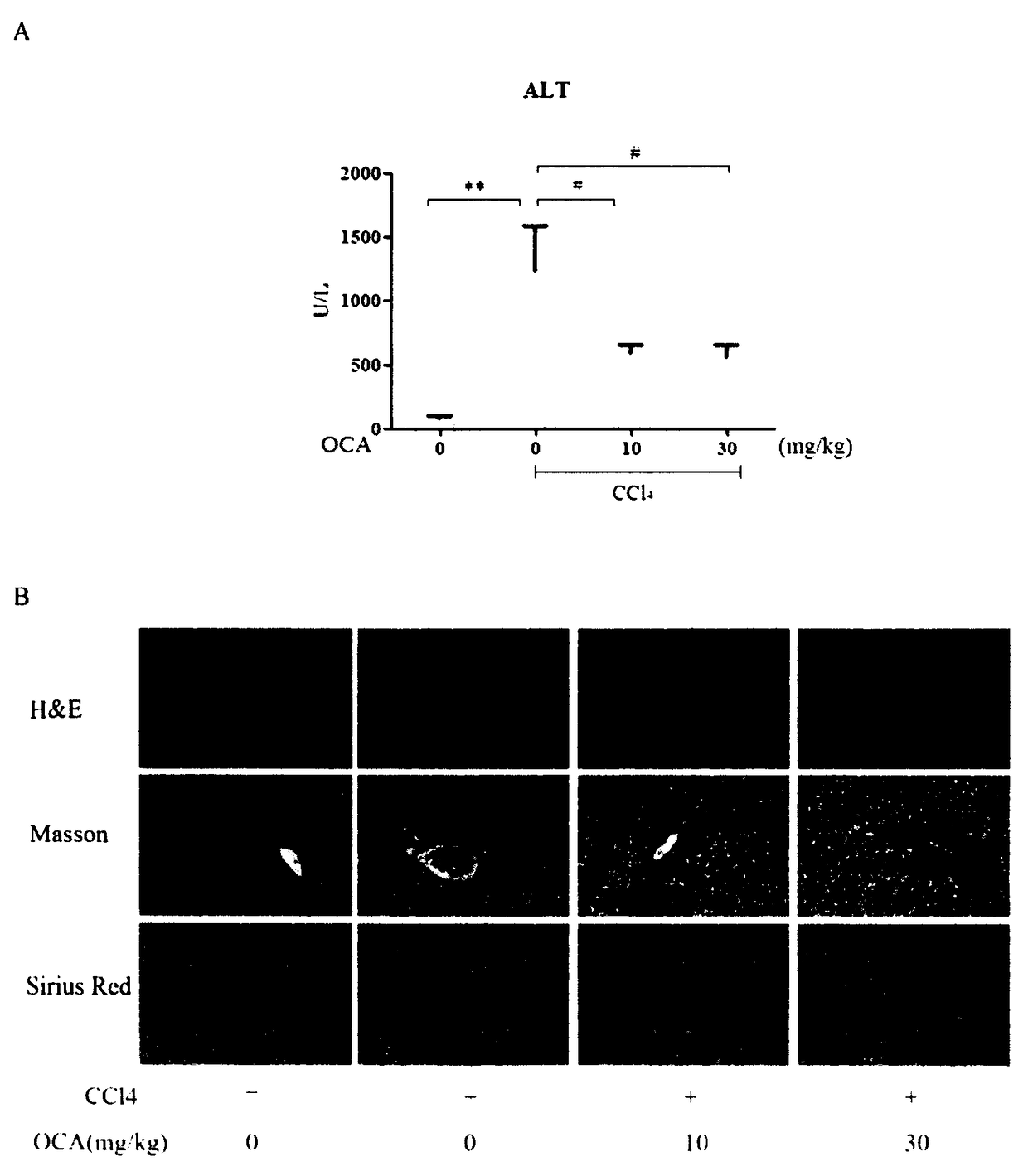
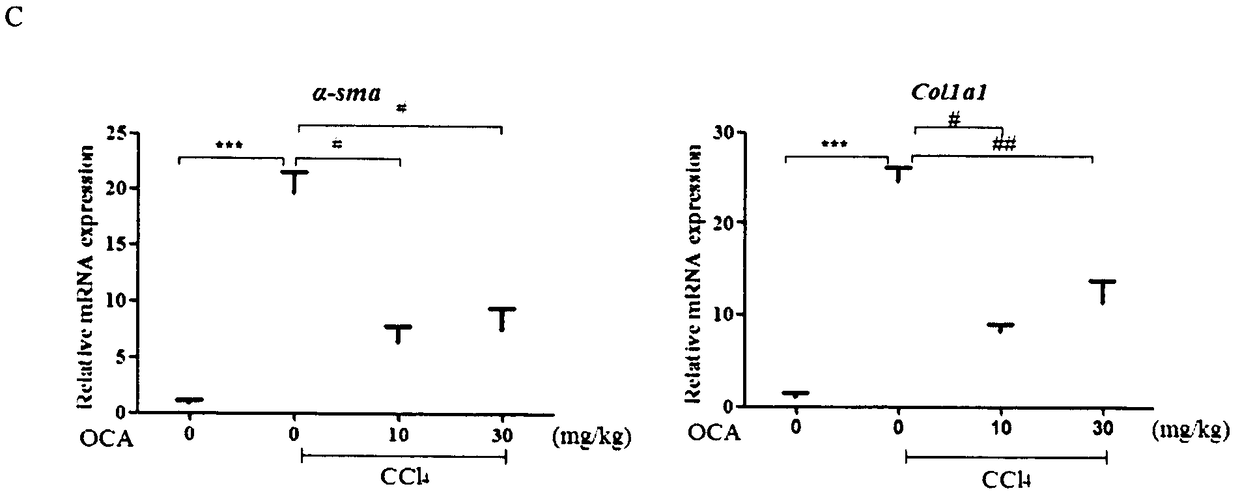
[0020] Experimental example 1 Obeticholic acid relieves CCl 4 Liver fibrosis induced by intraperitoneal injection
[0021] 1 Experimental materials
[0022] The C57BL / 6 species mice used in the present invention were purchased from the Comparative Medicine Center of Yangzhou University;
[0023] Obeticholic acid used in the present invention is purchased from MedChem Company, CCl 4 Purchased from Shanghai Lingfeng Chemical Reagent Company, mineral oil (Mineral Oil) purchased from Sigma-Aldrich Company; reverse transcription kit was purchased from Applied Biosystems Company, Trizol RNAiso plus was purchased from TAKARA Company.
[0024] 2 Experimental methods
[0025] 2.1 Establishment of animal model and administration method
[0026] 30 male C57BL / 6 mice (6-8 weeks old, body weight 20-24g) were randomly divided into blank group after adaptive feeding for 1 week, CCl 4 Model group, low-dose obeticholic acid group, and high-dose obeticholic acid group. Before the experime...
Embodiment 2
[0057] Example 2 The combination of obeticholic acid and IDN-6556 can significantly relieve CCl 4 Liver fibrosis induced by intraperitoneal injection
[0058] 1 Experimental materials
[0059] The IDN-6556 used in the present invention was purchased from MedChem, and other biological materials and chemical materials were the same as in Example 1.
[0060] 2 Experimental methods
[0061] 2.1 Establishment of animal model and administration method
[0062] 48 male C57BL / 6 mice (6 to 8 weeks, body weight 20g to 24g) were randomly divided into blank group after adaptive feeding for 1 week, CCl 4 The model group, the obeticholic acid single administration group, the IDN-6556 single administration group, and the obeticholic acid combined with IDN-6556 administration group consisted of 5 groups. Before the experiment, the animals had free access to food and maintained a circadian rhythm of 12 hours of light and 12 hours of darkness. Laboratory temperature: 20-25°C, humidity 50±5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com