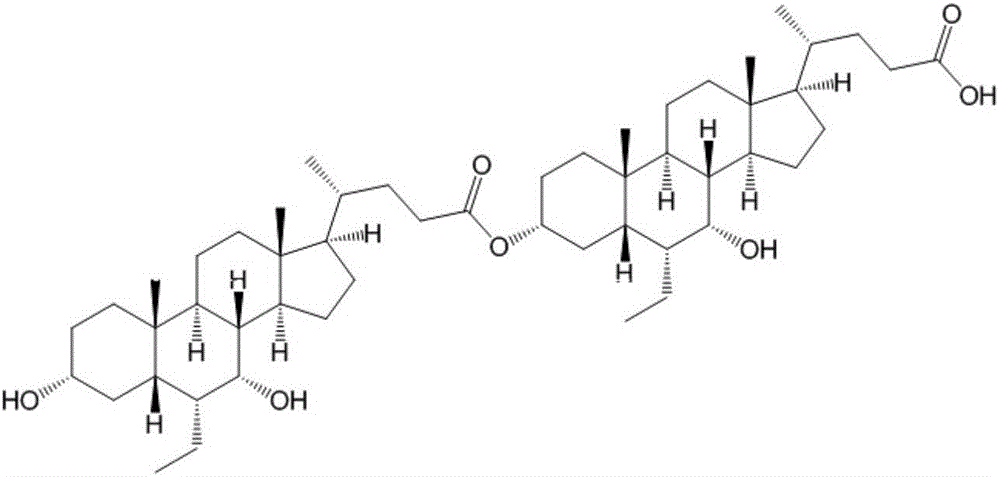
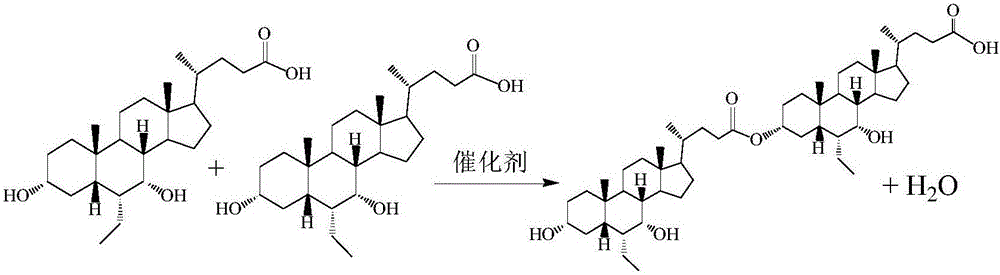
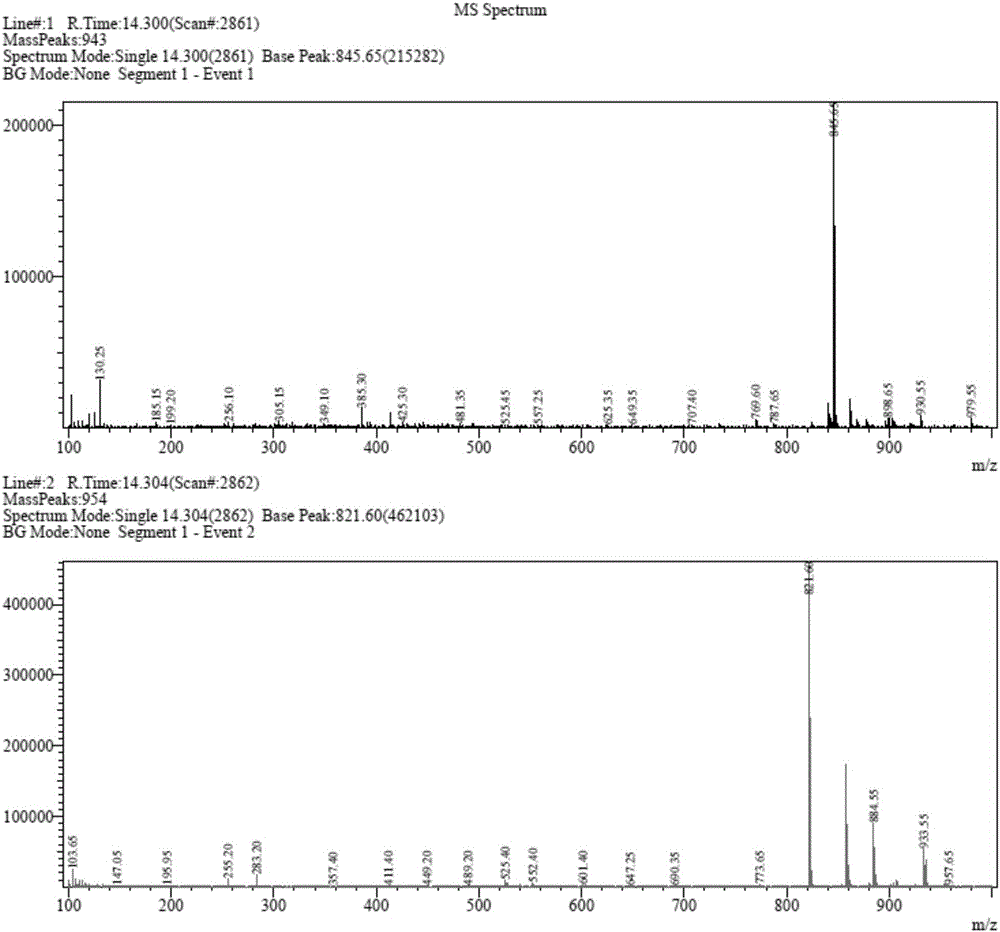
Obeticholic acid dimer impurities and preparation method thereof
A technology of obeticholic acid dimer and obeticholic acid, which is applied in the field of medicinal chemistry, can solve the problems of undisclosed preparation methods of obeticholic acid dimers and undisclosed preparation methods of dimer impurities, and achieve good The effects of control, high product purity, and improved safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0043] A preparation method of obeticholic acid dimer, comprising the steps of following successively:
[0044] S1: Preparation of a crude compound of obeticholic acid dimer impurity; it comprises the following sub-steps performed in sequence:
[0045] S1-1: adding obeticholic acid to 20-40 volume times of n-hexane or toluene or petroleum ether,
[0046] S1-2: Stir and heat reflux with water, keep the reaction at 65~120℃ for 4~8h, when the amount of water collected by weighing is about 80% of the theoretical water amount, cool down to 30~35℃, the theoretical water amount=input The weight of obeticholic acid ÷ 420.63 ÷ 2 × 18;
[0047] S1-3: add 5% sodium bicarbonate solution, stir and wash for 10 minutes, stand for stratification;
[0048] S1-4: the organic layer is evaporated to dryness under reduced pressure to obtain the crude compound of obeticholic acid dimer impurity;
[0049] S2: column chromatography separation and purification of obeticholic acid dimer impurity cru...
Embodiment 1
[0056] Embodiment 1 (best embodiment):
[0057] In a three-necked flask equipped with a stirrer, a water separator, and a thermometer, add 12 g of obeticholic acid, 360 ml of n-hexane, and 1 drop of concentrated sulfuric acid, reflux and dehydrate at 70 ± 2 °C for 6 h, cool to room temperature, and add 100 ml of water to wash , the aqueous layer was discarded, washed with 50 ml of 5% sodium bicarbonate solution, the aqueous sodium bicarbonate layer was discarded, and the organic layer was decompressed to dryness at 50 ° C to obtain the crude compound of obeticholic acid dimer impurity; Add 55 ml of petroleum ether to dissolve 11 g of the crude compound of cholic acid dimer impurity, add 20 g of 200-300 mesh silica gel, and rotate to steam until the solid in the bottle is quicksand. At the same time, 250g of 200-300 mesh silica gel was added to 500ml of petroleum ether as the stationary phase, and then added to the glass chromatography column after stirring evenly, and the glas...
Embodiment 2
[0058] Embodiment 2 (preferred embodiment):
[0059] In a three-necked flask equipped with a stirrer, a water separator, and a thermometer, add 12 g of obeticholic acid, 360 ml of toluene, and 1 drop of phosphoric acid, reflux and dehydrate at 112 ± 2 °C for 6 h, cool to room temperature, add 100 ml of water to wash, discard The aqueous layer was removed, washed with 50 ml of 5% sodium bicarbonate solution, the aqueous sodium bicarbonate layer was discarded, and the organic layer was decompressed to dryness at 55°C to obtain the crude compound of obeticholic acid dimer impurity; Add 55 ml of petroleum ether to dissolve 12 g of the dimer impurity crude compound, add 20 g of 200-300 mesh silica gel, and rotate to steam until the solid in the bottle is quicksand. At the same time, 250g of 200-300 mesh silica gel was added to 500ml of petroleum ether as the stationary phase, and then added to the glass chromatography column after stirring evenly, and the glass chromatography colum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com