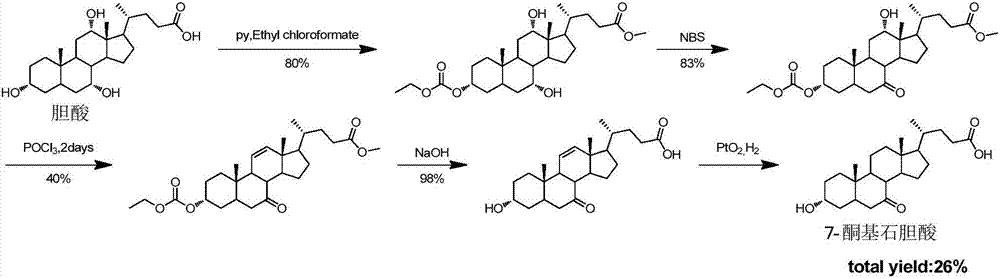
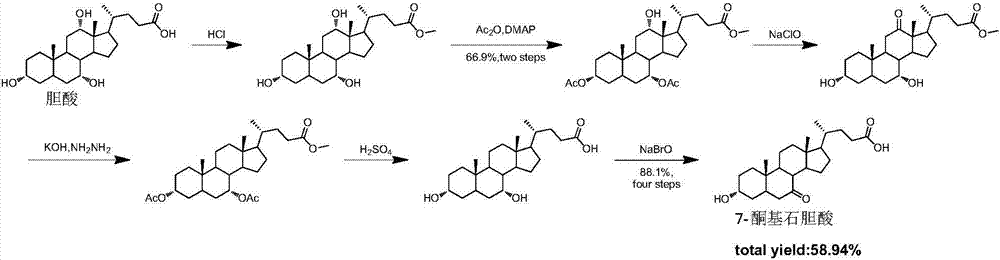
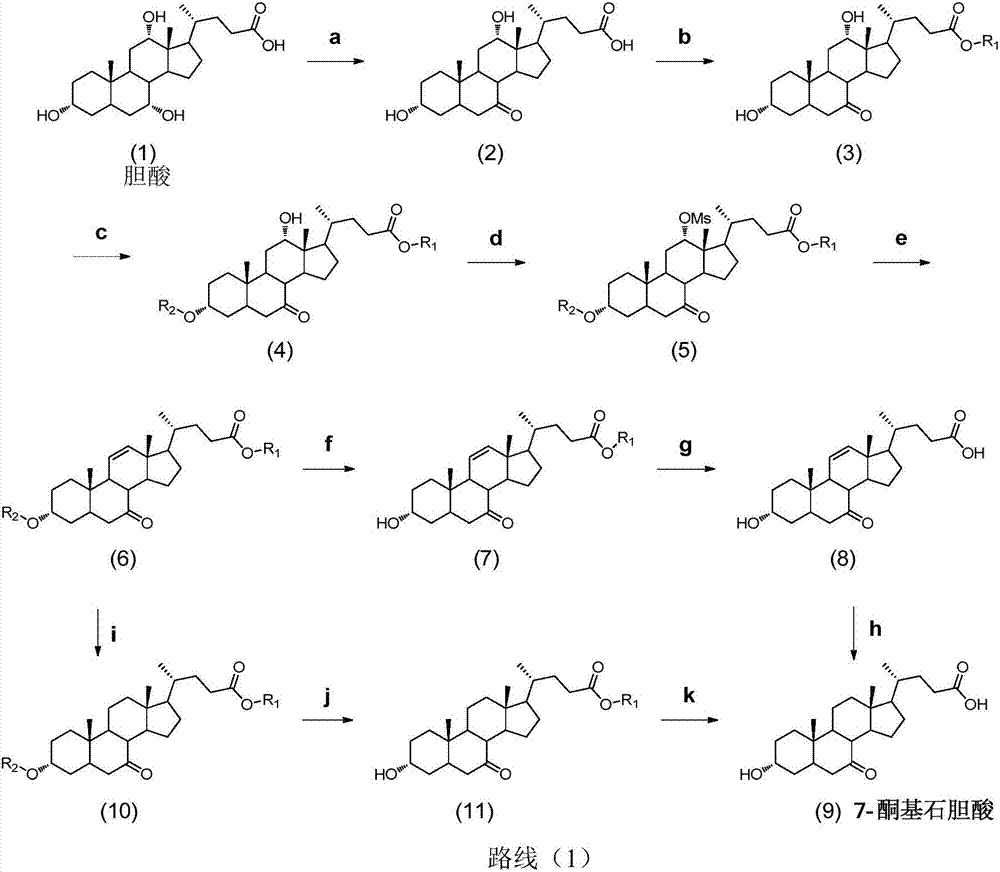
Synthesis method for obeticholic acid intermediate 7-ketolithocholic acid
A technology of obeticholic acid and a synthesis method, which is applied in the field of organic chemical synthesis and can solve the problems of high equipment requirements, low yield, and unsuitability for large-scale production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112]
[0113] 1. Synthesis of the compound of formula (2): Dissolve cholic acid (9g, 22.0mmol) in a mixed solution of 200mL acetone and water (v / v=3:1), avoid light, slowly add NBS (5.7g, 31.9 mmol), react at room temperature 25°C for 2h. After TLC detected that the reaction was complete, add 100mL saturated sodium bisulfite solution to quench the reaction, remove the solvent under reduced pressure until a white solid appeared, and pour it into 1L of water to precipitate a large amount of white solid, leave it to crystallize, filter and dry, The compound of formula (2) was obtained (8.5 g white solid, yield 95%). used directly in the next step.
[0114]
[0115] 2. Synthesis of the compound of formula (3-1): Dissolve the compound of formula (2) (8.5g, 20.9mmol) in 100mL of methanol, add concentrated sulfuric acid (0.11mL, 2.09mmol), and reflux for 2h. After the reaction was complete as detected by TLC, methanol was removed under reduced pressure, 30 mL of water was a...
Embodiment 2
[0129]
[0130] 1. Synthesis of the compound of formula (2): Dissolve 9g of cholic acid in a mixed solution of 200mL of acetone and water (v / v=3:1), keep away from light, and slowly add 5.7g of NBS (31.9mmol) after it is completely dissolved , room temperature reaction 2h. Add 100mL of saturated sodium bisulfite solution to prevent further oxidation, spin the solvent, when a white solid appears, pour it into clear water, a large amount of white solid precipitates, leave it to crystallize, and after suction filtration and drying, the compound of formula (2) ( 8.5g white solid, yield 95%), used directly in the next step.
[0131]
[0132] 2. Synthesis of the compound of formula (3-1): the compound of formula (2) (8.5 g, 20.8 mol) was dissolved in 100 mL of methanol, 1 mL of concentrated sulfuric acid was added, and the reaction was refluxed for 2 h. After the reaction was complete as detected by TLC, the solvent was evaporated under reduced pressure, 30 mL of water was ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com