Preparation for improving release efficiency
A technology of release efficiency and preparation, applied in the field of obeticholic acid dispersible tablets and its preparation, can solve problems such as large fluctuation range of tablet content, migration of soluble components of drugs, and influence on product efficacy, so as to increase solubility and dissolution rate, Enhance drug compliance and improve bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
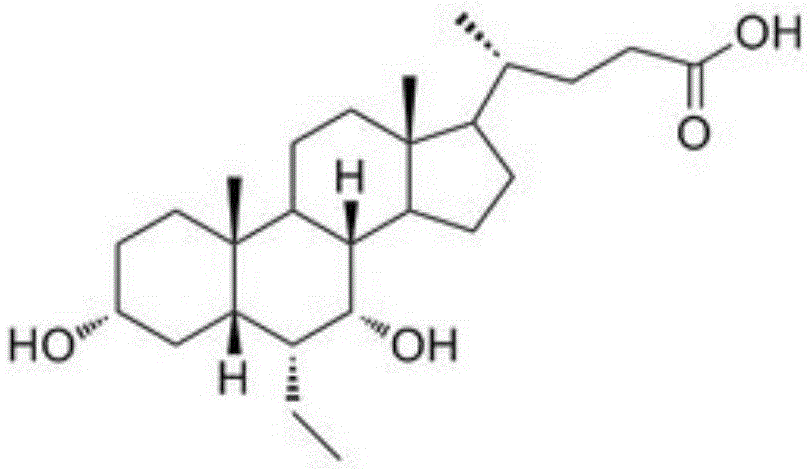
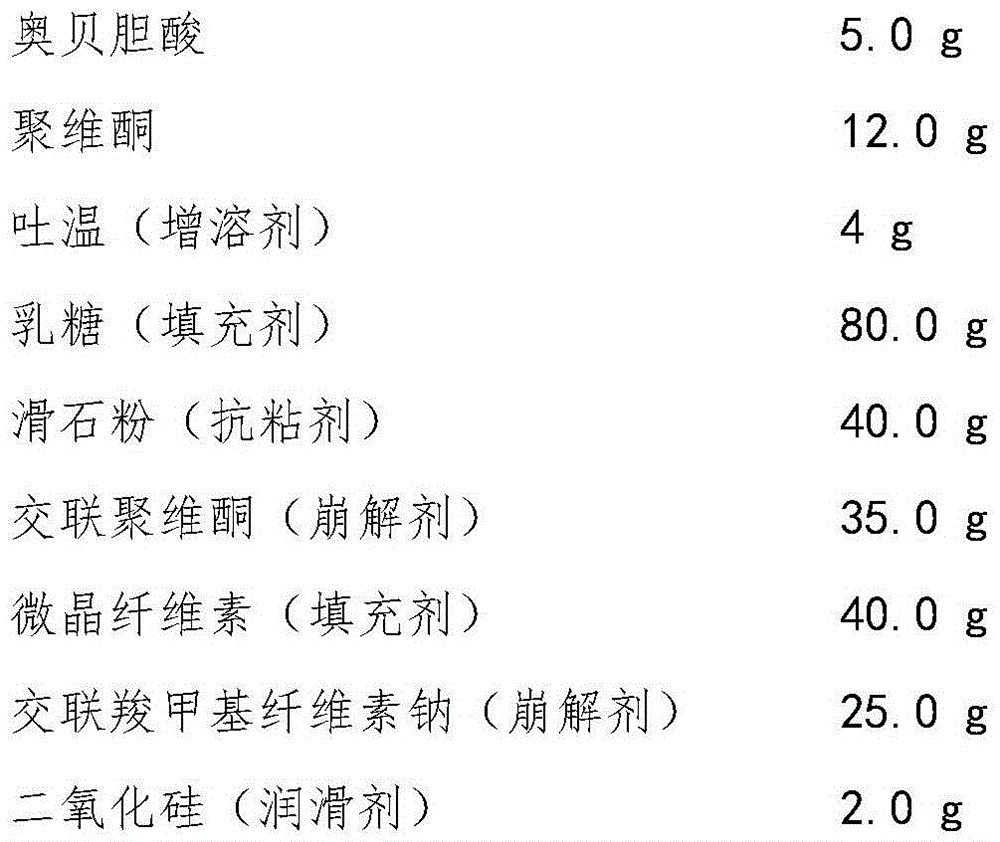
[0027] Obeticholic Acid Dispersible Tablets are prepared from the following components, and the dosage is 1000 tablets:
[0028]
[0029] Its preparation method comprises the following steps:
[0030] (1) Take obeticholic acid, dissolve it in absolute ethanol, then add povidone, stir vigorously to dissolve it into a clear and transparent solution, then add Tween, and continue stirring for 0.5-3h to obtain obeticholic acid solution. The obeticholic acid solution is prepared into a dry obeticholic acid solid dispersion by spray drying;
[0031] (2) Gradually add diluent, disintegrant, anti-adhesive agent, lubricant, and glidant by equal-volume incremental method. After repeated sieving and mixing, directly compress into tablets to obtain obeticholic acid dispersible tablets.
[0032] test results:
[0033] Compressibility: good
[0034] Appearance: smooth and beautiful
[0035] Uniformity of dispersion: in compliance with regulations
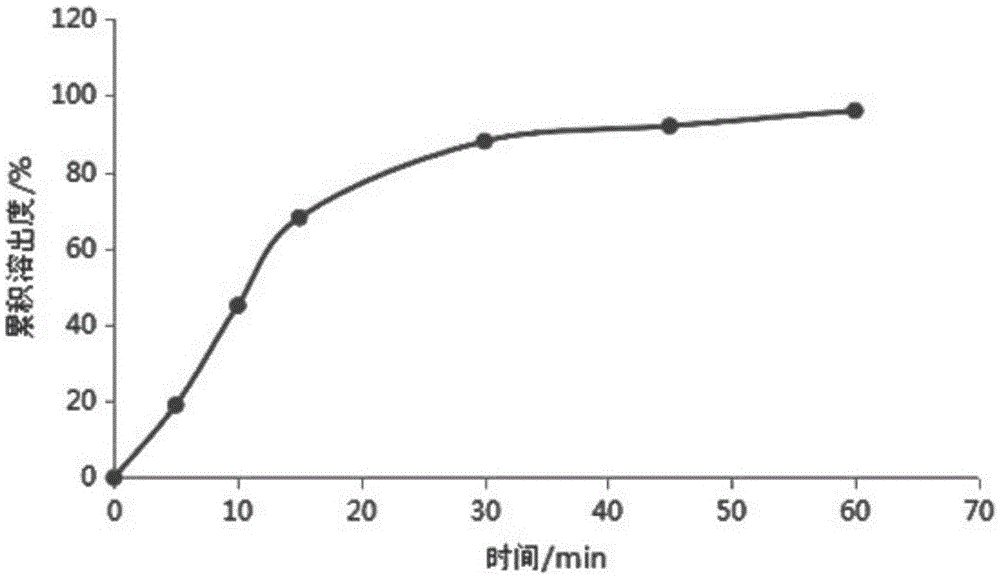
[0036] Average dissolution rate: 26...
Embodiment 2
[0038]
[0039] Its preparation method comprises the following steps:
[0040] (1) Take obeticholic acid, dissolve it in absolute ethanol, then add povidone, stir vigorously to dissolve it into a clear and transparent solution, then add polyoxyethylene hydrogenated castor oil, and continue stirring for 0.5-3h to obtain obeticholic acid acid solution, the obeticholic acid solution is prepared into a dry obeticholic acid solid dispersion by spray drying;
[0041] (2) Gradually add diluent, disintegrant, anti-adhesive agent, lubricant, and glidant by equal-volume incremental method. After repeated sieving and mixing, directly compress into tablets to obtain obeticholic acid dispersible tablets.
[0042] test results:
[0043] Compressibility: good
[0044] Friability: in compliance with regulations
[0045] Appearance: smooth and beautiful
[0046] Uniformity of dispersion: in compliance with regulations
[0047] Average dissolution rate: 37%
Embodiment 3
[0049]
[0050]
[0051] Its preparation method comprises the following steps:
[0052] (1) Take obeticholic acid, dissolve it in absolute ethanol, then add povidone, stir vigorously to dissolve it into a clear and transparent solution, then add potassium oleate, and continue stirring for 0.5-3h to obtain obeticholic acid solution, The obeticholic acid solution is prepared into a dry obeticholic acid solid dispersion by spray drying;
[0053] (2) Gradually add diluent, disintegrant, anti-adhesive agent, lubricant, and glidant by equal-volume incremental method. After repeated sieving and mixing, directly compress into tablets to obtain obeticholic acid dispersible tablets.
[0054] test results:
[0055] Compressibility: good
[0056] Friability: in compliance with regulations
[0057] Appearance: smooth and beautiful
[0058] Uniformity of dispersion: in compliance with regulations
[0059] Average dissolution rate: 50%
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com