Method for preparing obeticholic acid and intermediate thereof
A technology for obeticholic acid and intermediates, which is applied in the field of preparation of obeticholic acid and its intermediates, can solve the problems of low separation yield, achieve the effects of reducing side reactions, mild reaction conditions, and suitable for large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
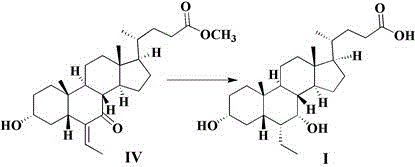
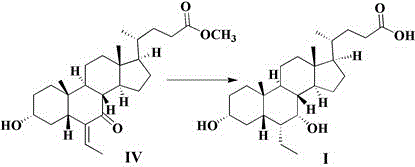
[0041] Embodiment 1: the preparation of compound III
[0042] Add 10.0g of compound IV and 100ml of methanol to the reaction flask, add 1.3g of sodium borohydride, keep warm at 20-40°C for 5 hours, concentrate to dryness under reduced pressure, add 100ml of water and 100ml of ethyl acetate, separate liquid, anhydrous sodium sulfate The organic phase was dried, filtered and concentrated to obtain 9.8 g of compound III with a yield of 97.6%.
[0043] MS (ESI, m / z): 433.6 ([M+H] + )
[0044] H-NMR: 5.556(d,1H), 4.483(d,1H), 4.138(d,1H), 3.736(s,1H), 3.573(s,3H), 3.403(s,1H), 2.290~2.401( m,2H), 2.177~2.237(m,1H), 0.839~1.945(m,27H), 0.699(s,3H), 0.585(s,3H).
Embodiment 2
[0045] Embodiment 2: the preparation of intermediate II
[0046] Dissolve 5.0g of compound III in 40ml of methanol, add 0.5g of palladium carbon (10wt%), react for 5h under hydrogen pressure of 1-2MPa and 20-40°C, and obtain 4.8g of intermediate II after filtration and concentration, with a yield of 95.6%.
[0047] MS (ESI, m / z): 435.6 ([M+H] + )
[0048] H-NMR: 4.028(m,2H), 3.573(s,3H), 3.493(s,1H), 3.126(t,1H), 2.299~2.342(m,1H), 2.191~2.232(m,1H), 1.902(d,1H), 1.670~1.815(m,7H), 0.875~1.527(m,17H), 0.845(d,3H), 0.821(d,6H), 0.602(s,3H).
Embodiment 3
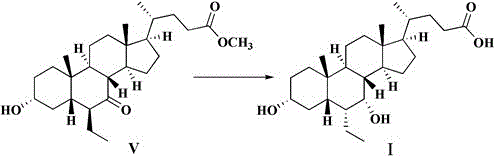
[0049] Example 3: Preparation of Obeticholic Acid (Compound I)
[0050] Dissolve 4.0 intermediate II in 16ml of methanol, add 8ml of 10% sodium hydroxide aqueous solution, react at 20-40°C for 1h, concentrate under reduced pressure, evaporate the organic solvent, add 40ml of purified water and 40ml of dichloromethane, and stir for 10-20min , separated, and the aqueous phase was washed twice with 40 ml of dichloromethane. Use 3mol / L hydrochloric acid to adjust the pH value of the aqueous phase to 7~8, add 40ml ethyl acetate, continue to use 3mol / L hydrochloric acid to adjust the pH value to 2~3, separate the liquids, and use 40ml water and 40ml saturated chlorine for the organic phase respectively. washed with sodium chloride, and the organic phase was dried over anhydrous sodium sulfate. Physic agent ammonium iyi was suction-filtered, the filtrate was concentrated under reduced pressure to near-dryness, and 2.2 g of obeticholic acid was refined by adding 80 ml of dichlorometh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com