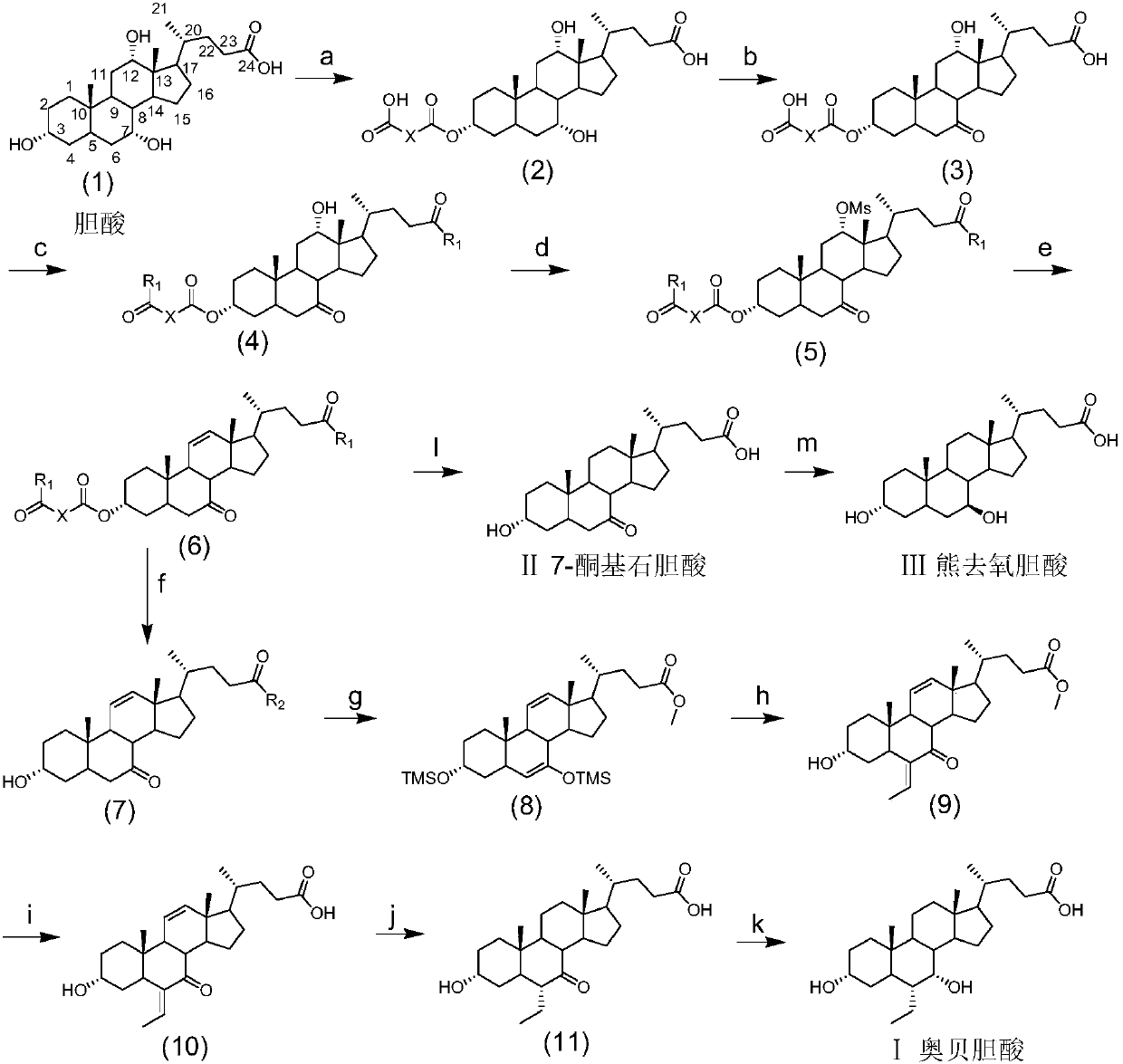
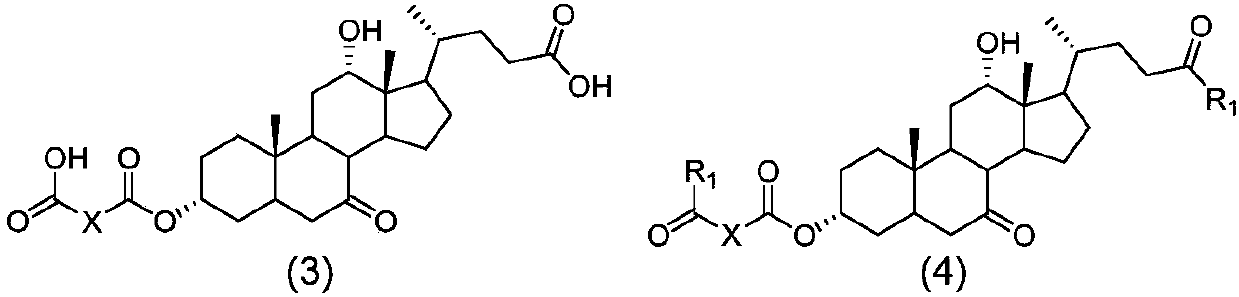
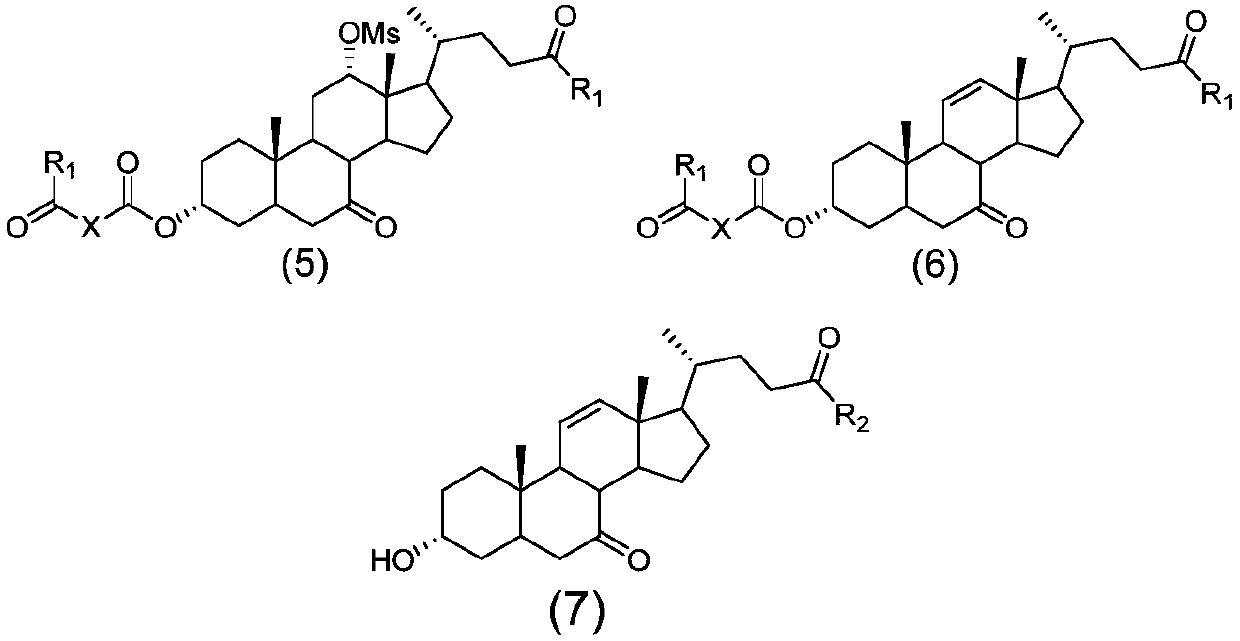
Method for preparing obeticholic acid, ursodeoxycholic acid and 7-ketolithocholicacid
A technology of ursodeoxycholic acid and obeticholic acid, which is applied in the production of steroids, organic chemistry, and bulk chemicals, can solve the problems of unsuitability for large-scale production, high equipment requirements, and low total yield, and achieve Facilitate industrial production, environmental friendliness and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0153] 1, the synthesis of formula (2-1) compound
[0154]
[0155] Cholic acid (10.00 g, 24.5 mmol) was dissolved in pyridine (50 mL), succinic anhydride (3.43 g, 34.3 mmol) was added, and the temperature was raised to 25° C. for 7 hours. After the reaction of the raw materials was detected by TLC, the reaction solution was cooled to room temperature, poured into 2M hydrochloric acid, stirred for 15 minutes, and suction filtered. The filter cake was washed with ether and dried to obtain the compound of formula (2-1) (12.5 g, white solid, 99 %). 1 HNMR (400MHz, DMSO-d 6 )δ12.07(s,2H),4.44(s,1H),4.03(d,J=7.2Hz,1H),3.79(s,1H),0.94(t,J=11.8Hz,5H),0.84( s,3H),0.59(s,3H).
[0156] 2, the synthesis of formula (3-1) compound
[0157]
[0158] The crude compound of formula (2-1) (12.50g, 24.5mmol) was dissolved in a mixed solvent of acetone (60mL) and water (60mL), added with NBS (5.7g, 31.9mmol), and reacted at 25°C for 2 hours. After the reaction of the raw materials was de...
Embodiment 2
[0193] 1, the synthesis of formula (2-2) compound
[0194]
[0195] Formula (1) cholic acid (4.08g, 10mmol) was dissolved in pyridine (50mL), glutaric anhydride (1.5g, 13mmol) was added, the temperature was raised to 80°C for 7 hours, and 300mg of glutaric anhydride was added for 7 hours. After the reaction of the raw materials was detected by TLC, the reaction liquid was cooled to room temperature, poured into 2M hydrochloric acid, and stirred for 15 min. After suction filtration, the filter cake was dried to obtain the compound of formula (2-2) (4.18 g, white solid, 82%). 1 H NMR (400MHz, CDCl 3 )δ4.59(s,1H),4.01(s,1H),3.87(s,1H),3.67(s,1H),1.00(d,J=5.6Hz,3H),0.90(s,3H), 0.70(s,3H).
[0196] 2, the synthesis of formula (3-2) compound
[0197]
[0198] The crude compound of formula (2-2) (4.18g, 8.2mmol) was dissolved in a mixed solvent of acetone (60mL) and water (20mL), NBS (2.3g, 13.3mmol) was added, and reacted in the dark for 2 hours. After the reaction of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com