Obeticholic acid and metformin composition and application thereof
A technology of metformin hydrochloride and obeticholic acid, applied in the field of medicine, can solve problems such as side effects of high and low density lipoprotein cholesterol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Therapeutic research experiment of abnormal blood index in golden hamster induced by high-fat diet
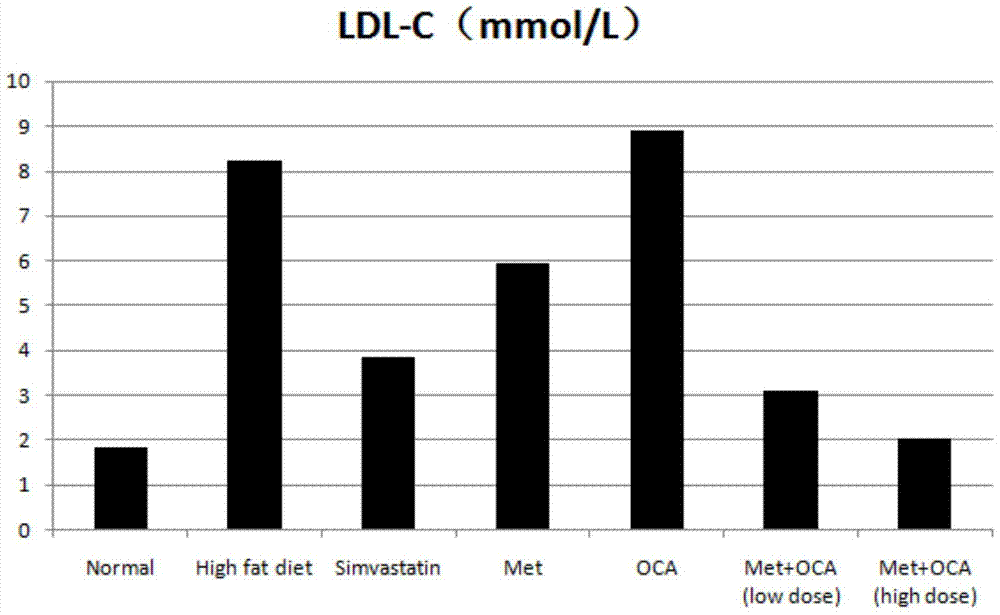
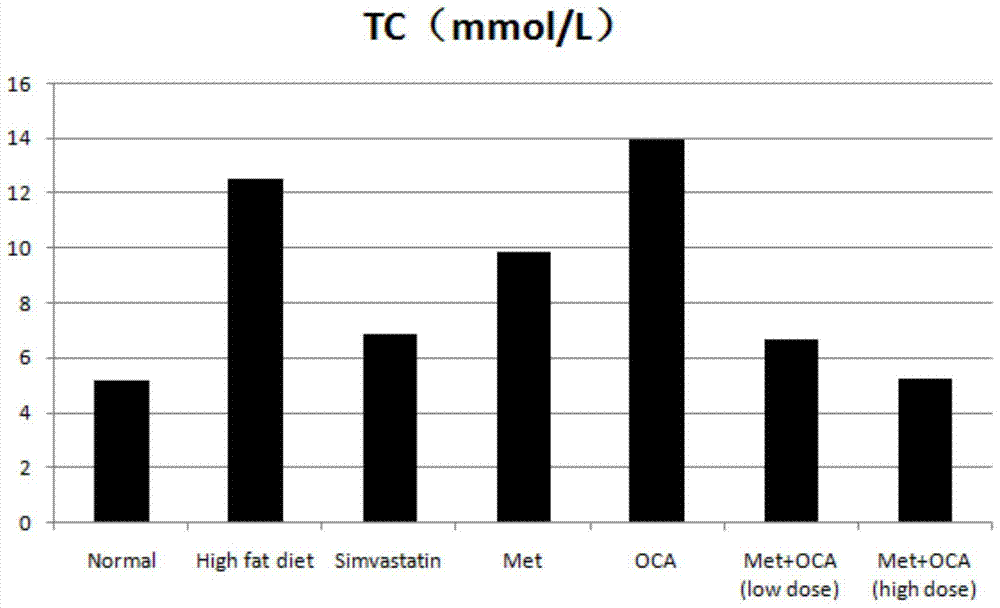
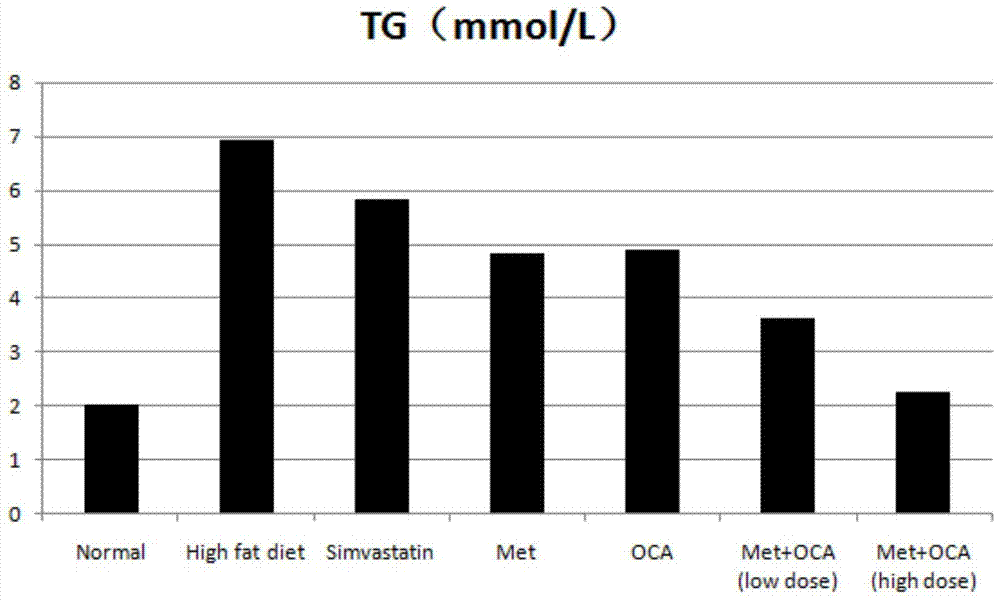
[0046] Model establishment: Golden hamsters were fed with custom-made high-fat diets, and normal rats were fed with normal rat growth feeds. Animal serum was collected after 4 weeks to detect changes in indicators. Compared with the golden hamsters in the normal group, the levels of LDL-C and TC in the serum of the golden hamsters induced by high-fat diet were significantly increased with statistical differences, which proved that the hyperlipidemia model was successfully established.
[0047] Test groups and dose design: normal control group, high-fat model control group, simvastatin group (8mg / kg), metformin (MET) group (100mg / kg), obeticholic acid (OCA) group (10mg / kg ), metformin+obeticholic acid (MET+OCA) low-dose group ((50mg+5mg) / kg), metformin+obeticholic acid (MET+OCA) high-dose group ((100mg+10mg) / kg). Number of animals: 10 per group. Administration...
Embodiment 2
[0057] Example 2 Study on Drug Curative Effect of Fatty Liver in Golden Hamsters Induced by High Fat Diet
[0058] Description of research animals: The research animals used in this experiment are the animals used in the above-mentioned "in vivo lipid-lowering experiment of the golden hamster model". Parts were frozen at -80°C, fixed for more than 48 hours, and examined for pathological section staining (H&E staining, Oil Red-O staining). Focus on the structural integrity of the liver, inflammatory cell infiltration, and the severity of fatty liver, and score them (grading criteria: grade 0: normal; grade 1: 80%). The results are shown in the table below.
[0059]
[0060] The results showed that in the high-fat model group, compared with the normal group, the volume of adipocytes in the liver increased significantly, and bullous steatosis appeared. A large number of inflammatory cells were infiltrated, and the above phenomena indicated that the modeling of non-alcoholic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com