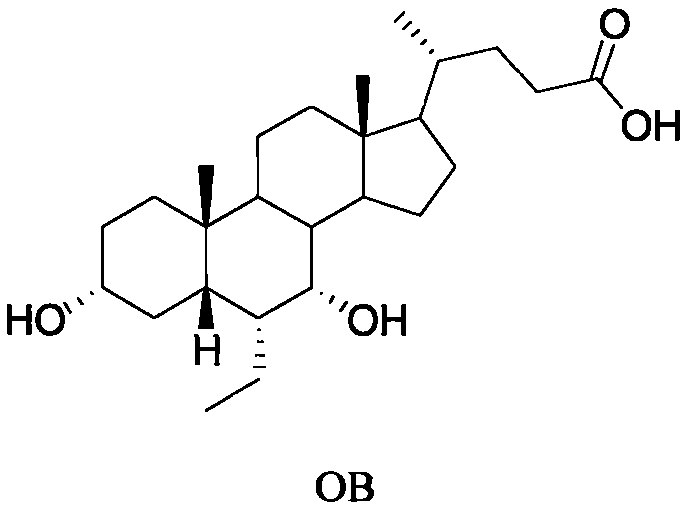
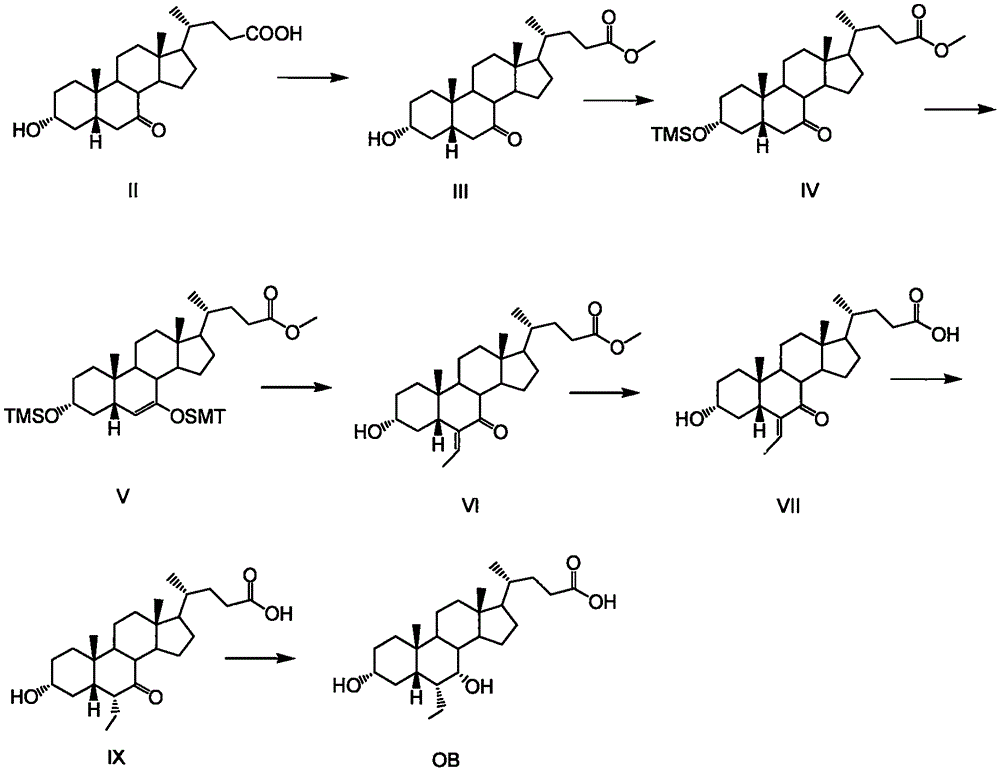
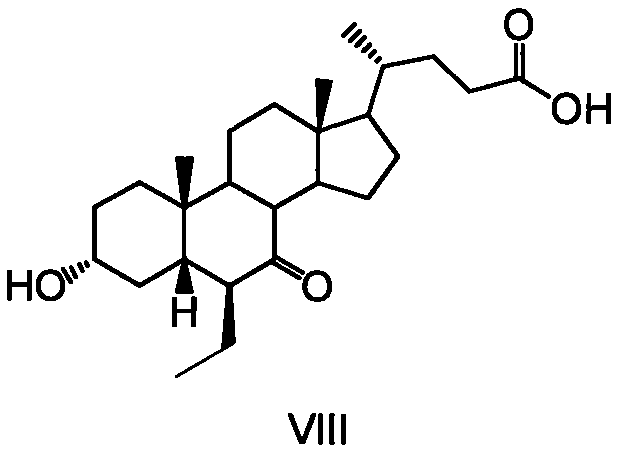
A method for preparing obeticholic acid and related compound
A technology of obeticholic acid and hydrochloric acid, which is applied in the field of medicine, can solve the problems of complex operation, difficulty in removing isomers, and high cost, and achieve the effects of improving refining efficiency, facilitating industrial production, and facilitating operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Preparation of E / Z-3α-tetrahydropyranyl hydroxy-6-ethylene-7-keto-5β-cholan-24-acid methyl ester (OB-3)
[0029] Weigh 200 g of E / Z-3α-hydroxy-6-ethylidene-7-keto-5β-cholane-24-acid methyl ester (OB-4) and dissolve it in 10 times the volume of dichloromethane, and cool to 0-5°C, add 78g of dihydropyran and 0.8g of p-toluenesulfonic acid in sequence, react for 8-10 hours, and the reaction of raw materials is complete. Add the reaction solution into 500mL of 5% sodium bicarbonate solution, stir for 15-30 minutes, and separate the organic phase; back-extract the water phase with 500mL of dichloromethane, combine the organic phases, wash with 500mL of water, and use 200g of anhydrous sodium sulfate for the organic phase dry. Filtration, the filtrate was concentrated to obtain the target product E / Z-3α-tetrahydropyranyl hydroxyl-6-ethylene-7-keto-5β-cholane-24-acid methyl ester (OB-3) 213g, yield 89.1% with a purity of 98.63%.
[0030] 1 H-NMR (CDCl 3 ): 6.11...
Embodiment 2
[0031] Example 2: Preparation of 3α-tetrahydropyranyl hydroxy-6β-ethyl-7-keto-5β-cholan-24-acid (OB-2)
[0032] Weigh 210 g of E / Z-3α-tetrahydropyranyl hydroxy-6-ethylene-7-keto-5β-cholan-24-acid methyl ester (OB-3) prepared in Example 1 and dissolve in Add 5 times the volume of MeOH to 10 times the volume of 2% NaOH solution, add 10 g of palladium carbon, hydrogenate at room temperature for 18-24 hours, heat the reaction solution to 90-95 ° C, and react for 2-4 hours. Most of the methanol was distilled off under reduced pressure, adjusted to PH=4-6 with 2N hydrochloric acid, extracted with 1L×2 ethyl acetate, combined organic phases, washed with 1L water, and dried with 250 g of anhydrous sodium sulfate. Filter and concentrate the filtrate. The residue was washed with ethyl acetate / n-heptane=1 / 1, filtered, and air-dried at 40-50°C to obtain the target product 3α-tetrahydropyranylhydroxy-6α-ethyl-7-keto-5β- Cholan-24-acid (OB-2) 197g, yield 93.4%, purity 98.95%, OB2-A 0.32%....
Embodiment 3
[0033] Example 3: Preparation of 3α-tetrahydropyranyl hydroxy-6α-ethyl-7α-hydroxyl-5β-cholan-24-acid (OB-1)
[0034] Weigh 195 g of 3α-tetrahydropyranyl hydroxy-6α-ethyl-7-keto-5β-cholan-24-acid (OB-2) prepared in Example 2 and dissolve in 10 times the volume of 2% NaOH In the solution, heat to 85-95°C, add 21g of sodium borohydride, react at 85-95°C for 5-8 hours, and the reaction of raw materials is complete. Cool to 40-50°C, adjust the pH to 4-6 with 2N hydrochloric acid, extract with 1L×2 ethyl acetate, combine the organic phases, wash with 1L water, and dry the organic phase with 250g of anhydrous sodium sulfate. Filter and concentrate the filtrate. The residue was washed with ethyl acetate / n-heptane=1 / 1, filtered, and air-dried at 40-50°C to obtain the target product 3α-tetrahydropyranylhydroxy-6α-ethyl-7α-hydroxy-5β-cholate Alkane-24-acid (OB-1) 177g, yield 90.7%, purity 99.16%, OB-1A content 0.06%.
[0035] 1 H-NMR (CDCl 3 ): 4.75(1H, br), 3.90-3.95(1H, m), 3.71(1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com