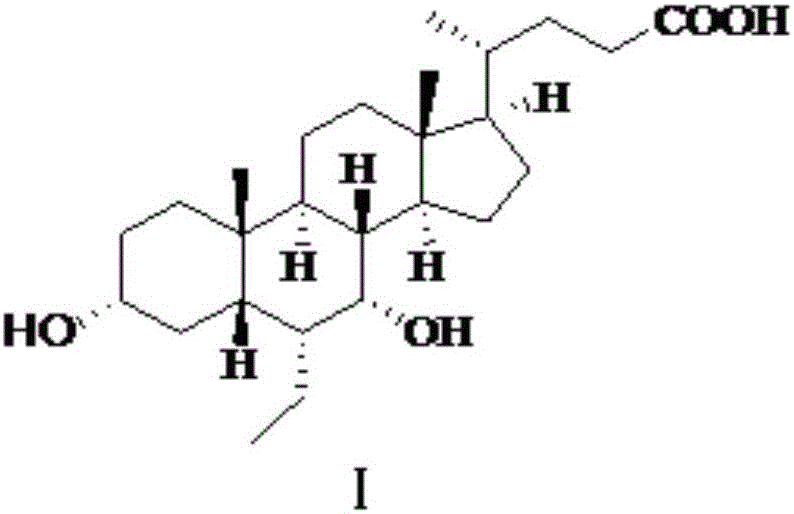
Synthesis method of obeticholic acid
A technology of obeticholic acid and a synthesis method, which is applied in the field of drug synthesis, can solve the problems of easy configuration inversion, many impurities, difficult purification and the like, and achieves the effects of improving the total yield, convenient purification and good economy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
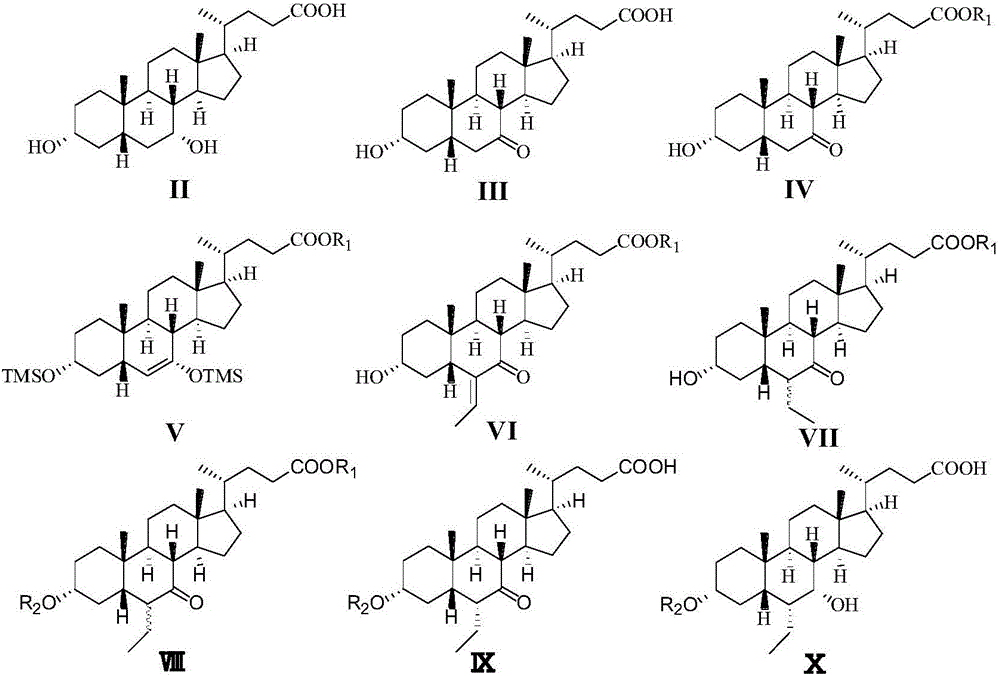
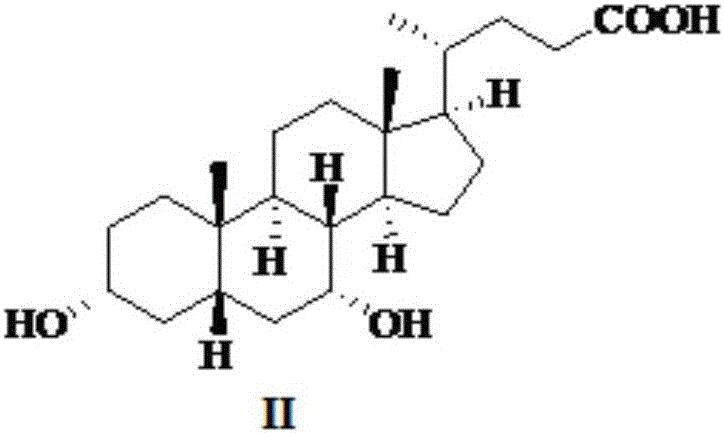
[0045] Preparation of 3α-Hydroxy-7-keto-5β-cholestane-24-acid (III)
[0046] Dissolve the compound (118.0 g, 0.31 mol) shown in formula (II) in dichloromethane (500 ml), add 30% sulfuric acid (80 ml) solution and sodium bromide (800 mg) and stir at room temperature, and control the temperature at 30 ° C Add 16% sodium bromate (162.4g, 1.06mol) solution dropwise, and react for 6h after the dropwise addition, add 10% sodium bisulfite solution (200ml), extract and retain the organic layer, and use ethyl acetate (200ml) to re- extraction. The organic phases were combined, concentrated to dryness, added ethyl acetate (200ml), heated to reflux for 0.5h, cooled down and crystallized for 24h, filtered, and the filter cake was air-dried at 50°C for 10h to obtain a white solid of the compound shown in formula (III) ( 112.5 g, yield 96.1%).
Embodiment 2
[0048] Preparation of 3α-hydroxy-7-keto-5β-cholestane-24-ethyl ester (IV)
[0049] The compound represented by formula (III) (100.0 g, 0.26 mol) was dissolved in ethanol (500 ml), concentrated sulfuric acid (5 ml) was added, heated to reflux for 10 h, and cooled to room temperature. Add 1mol / L sodium hydroxide solution (about 30ml) to adjust the pH to 7, slowly add purified water (1L) dropwise, stir at room temperature, a white solid precipitates, and let it stand overnight. After suction filtration, the filter cake was vacuum-dried at 50° C. to obtain a white solid of the compound represented by formula (IV) (105.5 g, 98.4%).
Embodiment 3
[0051] Preparation of 3α,7-bis(trimethylsilyloxy)-6-ene-5β-cholestane-24-ethyl ester formula (V)
[0052] Anhydrous THF (400ml), 2mol / LLDA THF solution (150ml) and trimethylchlorosilane (110ml, 0.9mol) were added to the reaction flask under nitrogen protection. Stir at -50~-30°C for 15min, slowly add dropwise anhydrous THF solution (100ml) containing the compound (80.3g, 0.2mol) shown in (IV), and add dropwise at -50~-30°C under temperature control. Keep stirring at this temperature for 6h, add saturated sodium bicarbonate solution (100ml) to quench the reaction. Slowly rise to room temperature, continue stirring for 2 h, add saturated sodium bicarbonate solution (1 L), extract with ethyl acetate (1 L × 2), combine organic layers, wash with water (1 L) and saturated sodium chloride solution (1 L) successively, After drying with anhydrous sodium sulfate and filtering, the filtrate was concentrated to dryness to obtain a tan solid (120.7 g) of the compound represented by formul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com