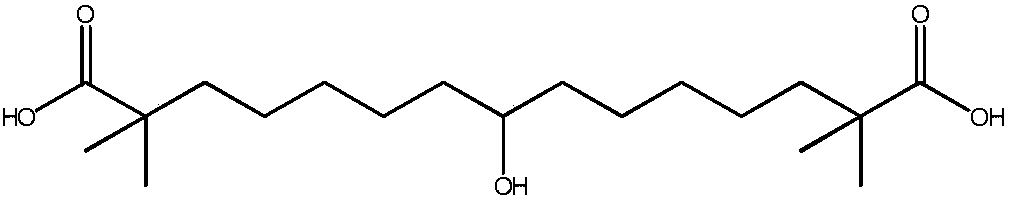
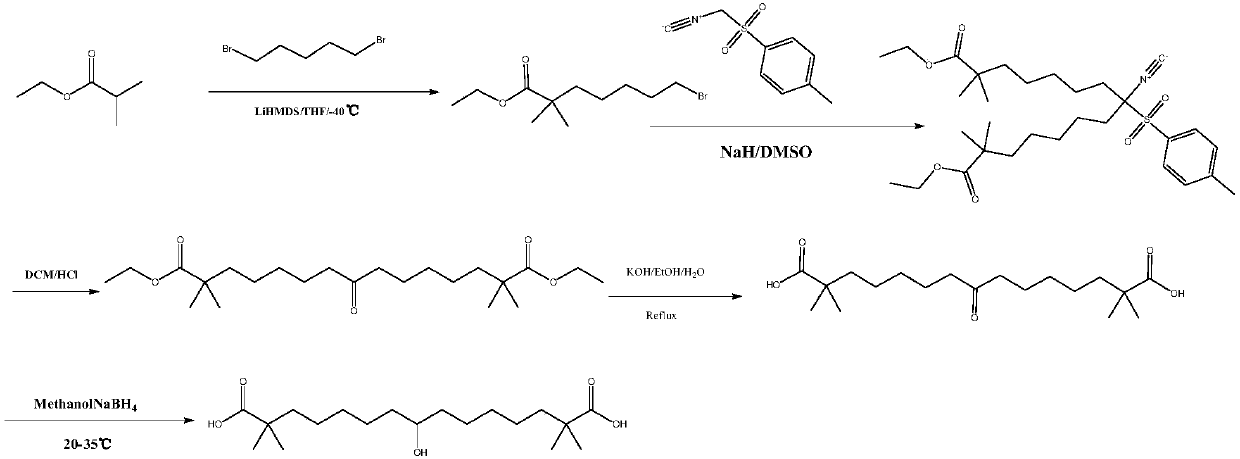
Synthetic method for 8-hydroxyl-2,2,14,14-tetramethyl-pentadecanedioic acid
A technology of pentadecanedioic acid and pentadecanedioic acid salt, applied in chemical instruments and methods, carboxylate preparation, carboxylate preparation and other directions, can solve the problem of not easy industrial production, incomplete reaction, high risk problems, to achieve the effect of reducing cost input, reducing risk, and simplifying operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Pour 5.0g (14.6mmol) of 8-keto-2,2,14,14-tetramethyl-pentadecanedioic acid into 50ml of purified water, add 1.46g (36.5mmol) of sodium hydroxide, and keep stirring until all Dissolve, react at 20°C for 30 minutes, add 0.55 g (14.6 mmol) of sodium borohydride to the reaction solution, continue the reaction for 4 hours, and spot the plate with TLC (developing agent: ethyl acetate: n-hexane: acetic acid = 1:5 :0.3) The reaction is complete. Add concentrated hydrochloric acid to the reaction solution, adjust the pH to 1, stir for 10 minutes, add 60ml of methyl tert-butyl ether for extraction three times, combine the organic layers, wash the solution with 60ml of purified water for three times, separate the organic layer, Wash once with 60ml of saturated brine, dry over anhydrous sodium sulfate for 2 hours, filter and evaporate the organic layer to dryness to obtain a white solid which is 8-hydroxy-2,2,14,14-tetramethyl-pentadecanedioic acid, Yield 97.9%, purity 99.3%.
Embodiment 2
[0027] Pour 5.0g (14.6mmol) of 8-keto-2,2,14,14-tetramethyl-pentadecanedioic acid into 75ml of purified water, add 2.46g (43.8mmol) of potassium hydroxide, and keep stirring until all Dissolved, reacted for 30 minutes at 25°C, added 0.66 g (17.5 mmol) of sodium borohydride to the reaction solution, continued the reaction for 4 hours, and spotted the plate with TLC (developing agent: ethyl acetate: n-hexane: acetic acid = 1:5 :0.3) The reaction is complete. Add conc Wash once with 60ml of salt water, dry over anhydrous sodium sulfate for 2 hours, filter and evaporate the organic layer to dryness to obtain a white solid, which is 8-hydroxy-2,2,14,14-tetramethyl-pentadecanedioic acid. Yield 96.3%, purity 98.5%.
Embodiment 3
[0029] Pour 5.0g (14.6mmol) of 8-keto-2,2,14,14-tetramethyl-pentadecanedioic acid into 60ml of purified water, add 2.16g (29.2mmol) of calcium hydroxide, and keep stirring until all Dissolve, react at 20°C for 30 minutes, add 0.55 g (14.6 mmol) of sodium borohydride to the reaction solution, continue the reaction for 4 hours, and spot the plate with TLC (developing agent: ethyl acetate: n-hexane: acetic acid = 1:5 :0.3) The reaction is complete. Add concentrated hydrochloric acid to the reaction solution, adjust the pH to 1, stir for 10 minutes, add 60ml of methyl tert-butyl ether for extraction three times, combine the organic layers, wash the solution with 60ml of purified water for three times, separate the organic layer, Wash once with 60ml of saturated brine, dry over anhydrous sodium sulfate for 2 hours, filter and evaporate the organic layer to dryness to obtain a white solid, which is 8-hydroxy-2,2,14,14-tetramethyl-pentadecanedioic acid , yield 95.9%, purity 98.2%. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com