Triadimefon and triadimenol compounds having antimicrobial activity, salts, synthetic methods and uses thereof
An antimicrobial and chemical compound technology, applied in the direction of organic active ingredients, chemicals for biological control, botany equipment and methods, etc., can solve serious problems such as drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
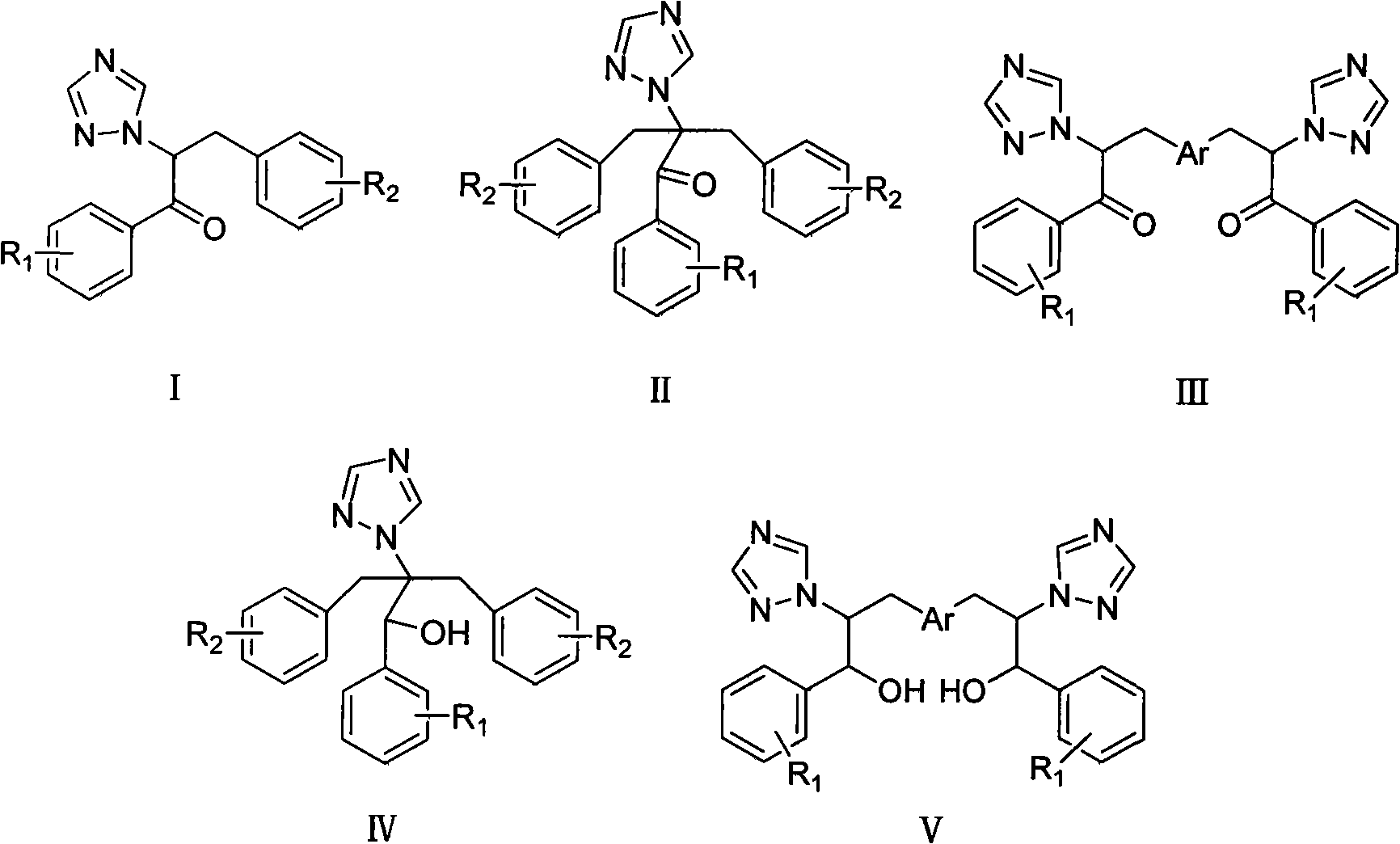
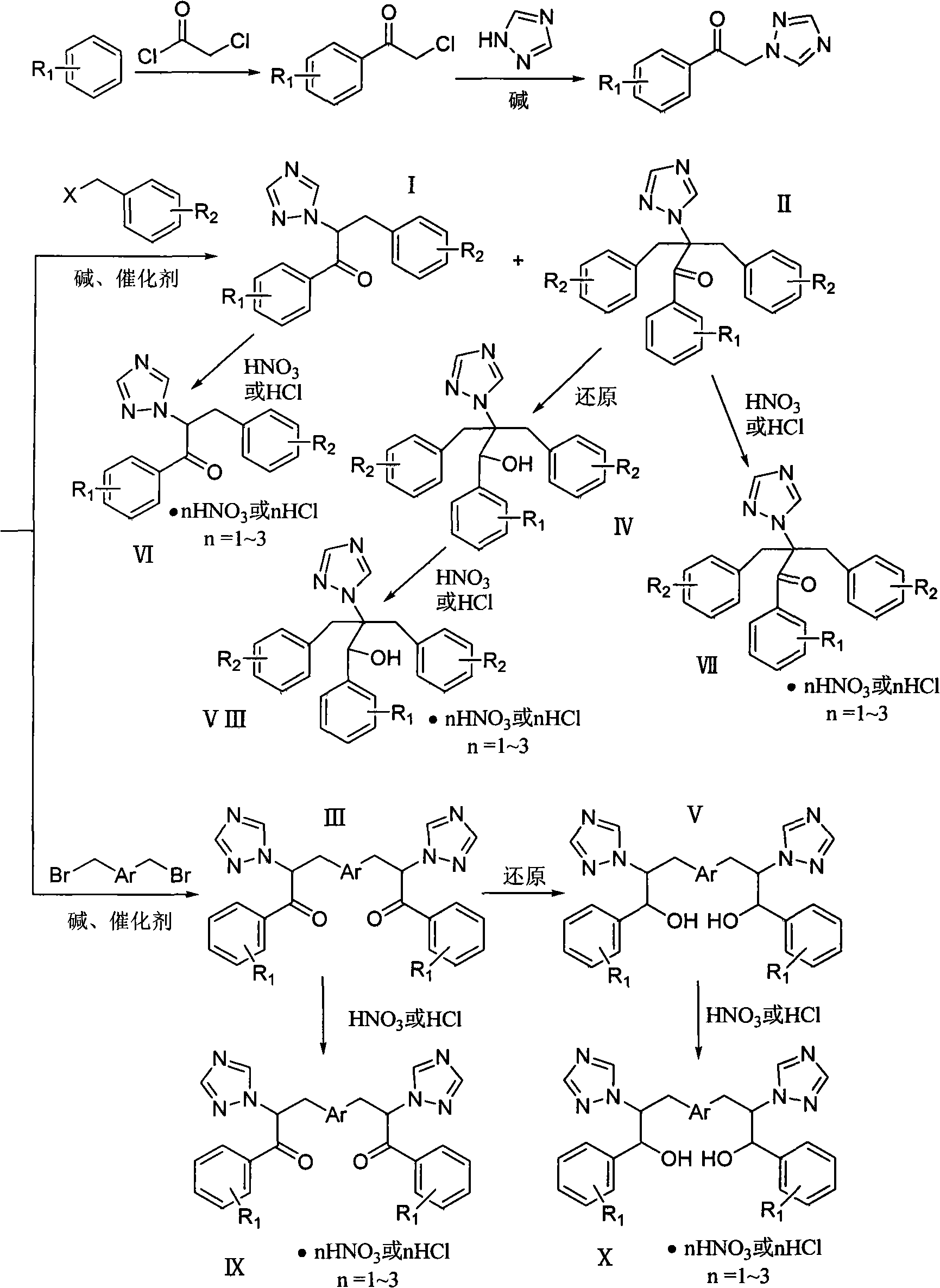
[0041] Example 1: Compound I-1: 3-(2,4-dichlorophenyl)-1-(2,4-difluorophenyl)-2-(1H-1,2,4-triazole)acetone
[0042] (0.88g, 0.004mol) 1-(2,4-difluorophenyl)-2-(1H-1,2,4-triazole) ethanone, potassium carbonate (0.70g, 0.005mol), tetrabutyl Ammonium bromide (25 mg), 20 mL of tetrahydrofuran were placed in a 150 mL three-necked round bottom flask, and stirred at room temperature for 1 h. (1.45 g, 0.007 mol) 2,4-dichlorobenzyl chloride was added, and the temperature of the oil bath was controlled at 45° C. and stirred for 8 h. During the reaction process, the acidity of the reaction solution was constantly checked to keep the pH value greater than 9. Use thin-layer chromatography (developer: chloroform / acetone 10 / 1, V / V) to track the reaction. After the reaction is stopped, distill off tetrahydrofuran, extract with chloroform (20mL×3), dry over anhydrous sodium sulfate, and concentrate the mother liquor to obtain the initial product , silica gel column chromatography (developer:...
Embodiment 2
[0057] Example 2: Compound II-1: 2-(2,4-dichlorophenyl)-3-(2,4-dichlorophenyl)-1-(2,4-difluorophenyl)-2- (1H-1,2,4-triazole)acetone
[0058] 1.17g, (0.005mol) 1-(2,4-difluorophenyl)-2-(1H-1,2,4-triazole) ethanone, sodium hydroxide (0.63g, 0.015mol), four Butylammonium bromide 50mg, 10mL tetrahydrofuran and 10mL H 2 O was placed in a 250mL three-neck round bottom flask and stirred at room temperature for 1h. (2.45 g, 0.013 mol) 2,4-dichlorobenzyl chloride was added, and the temperature of the oil bath was controlled at 45° C. and stirred for 6 h. During the reaction process, the acidity of the reaction solution was constantly checked to keep the pH value greater than 9. Use thin-layer chromatography (developer: chloroform / acetone 10 / 1, V / V) to track the reaction. When the reaction is complete, distill off tetrahydrofuran, extract with chloroform (20mL×3), dry over anhydrous sodium sulfate, filter, and concentrate the mother liquor to obtain 3.64 g of the initial product was...
Embodiment 3
[0071] Example 3: Compound III-1: 3,3'-(1,4-phenyl)bis(1-(2,4-difluorophenyl)-2-(1H-1,2,4-triazole )acetone
[0072] 1.17g (0.005mol) 1-(2,4-difluorophenyl)-2-(1H-1,2,4-triazole) ethanone, sodium hydroxide (0.63g, 0.015mol), tetrabutyl 50 mg of ammonium bromide, 10 mL of benzene and 10 mL of water were placed in a 250 mL three-necked round bottom flask, and stirred at room temperature for 1 h. Add (2.45 g, 0.013 mol) p-dibenzyl bromide, and stir in an oil bath at 45° C. for 6 h. During the reaction process, the acidity of the reaction solution was constantly checked to keep the pH value greater than 9. Use thin-layer chromatography (developer: chloroform / acetone 10 / 1, V / V) to track the reaction. When the reaction is complete, evaporate the solvent under reduced pressure, extract with chloroform (20mL×3), dry over anhydrous sodium sulfate, filter, and the mother liquor Concentrate to obtain 3.64 g of the initial product, and perform silica gel column chromatography (develope...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com