Method for catalytically synthesizing atazanavir intermediate
A technology of atazanavir and intermediates, applied in the direction of fixing on or in inorganic carriers, chemical industry, sustainable manufacturing/processing, etc., to achieve the effects of improving regeneration efficiency, reducing dosage, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
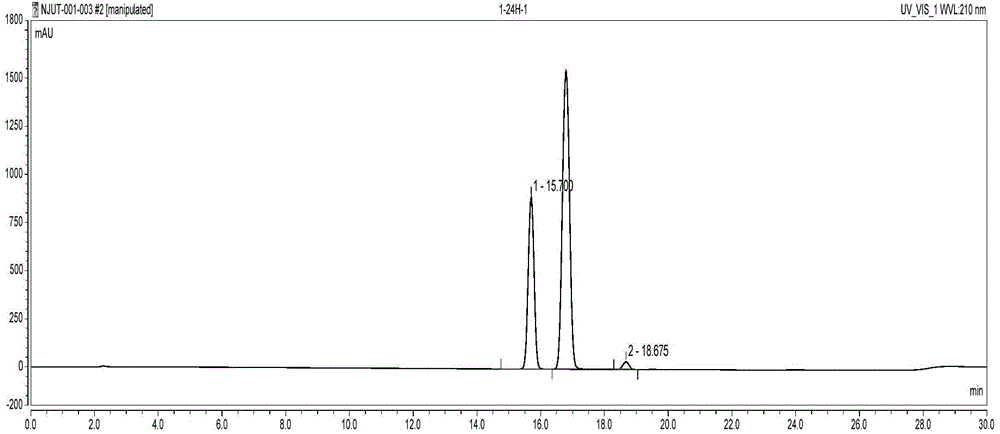
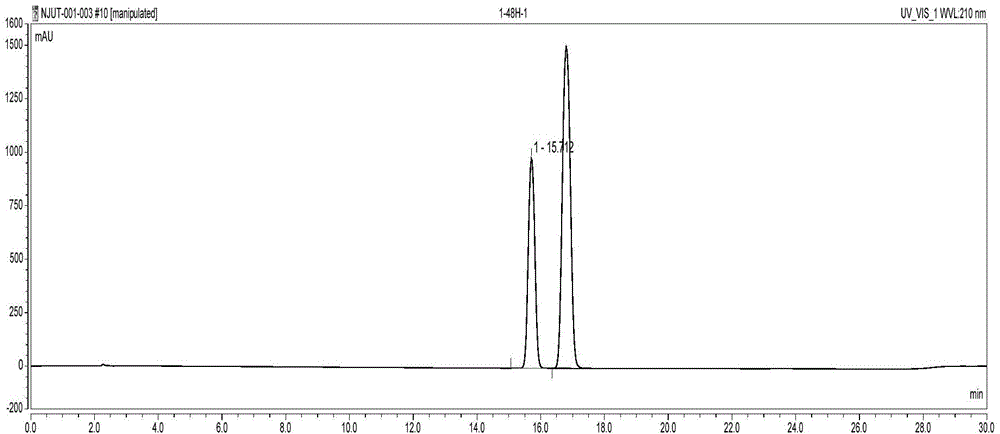
Image
Examples
Embodiment 1
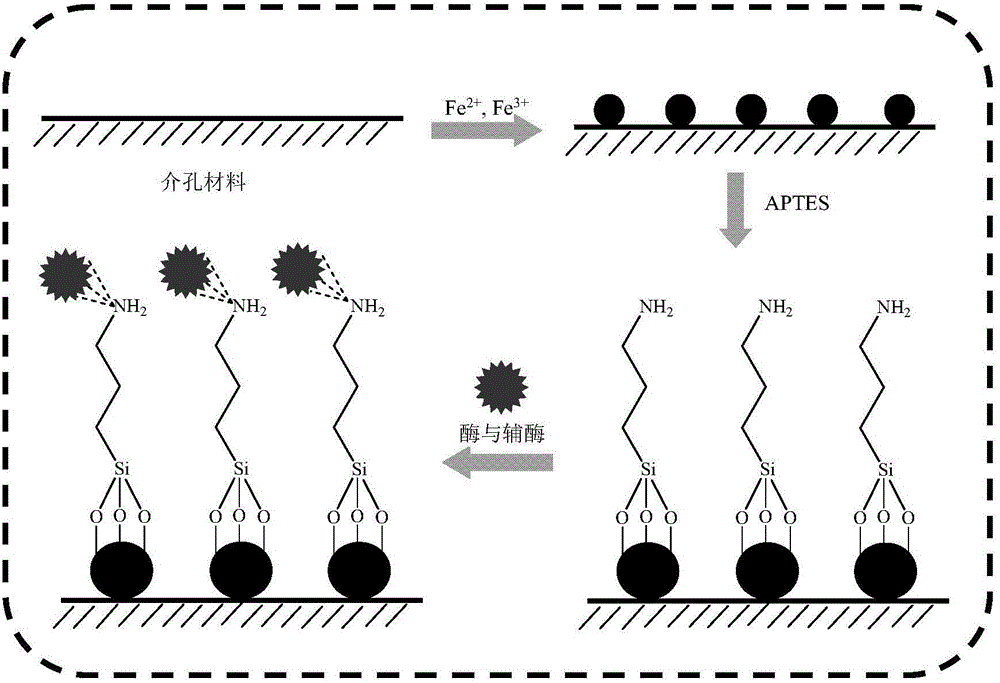
[0029] Example 1 This example illustrates the method for obtaining the modified magnetic mesoporous material
[0030] Weigh 1 g SBA-15 and add it to the content of 1.75 g (NH 4 ) 2 Fe(SO 4 ) 2 .6H 2 O, 4.29 g NH 4 Fe(SO 4 ) 2 .12H 2 In the aqueous solution of O, adjust the pH to 11~12 with 5 M ammonia water, stir at 65°C for 30 min, and magnetically adsorb to obtain the mesoporous material SBA-15 / Fe containing magnetism. 3 O 4 . Weigh 1 g SBA-15 / Fe 3 O 4 Add to 500 ml of ethanol aqueous solution (V / V=1:1), stir under the protection of argon, add glacial acetic acid to pH 4.0, add 50 ml of 3-aminopropyl triethoxy group dropwise to the system Silane, stirred at 60°C for 24 hours, magnetically adsorbed, and washed with ethanol three times to obtain the modified magnetic mesoporous material SBA-15 / Fe 3 O 4 -NH 2 .
Embodiment 2
[0031] Example 2 This example illustrates the method of obtaining a co-immobilized enzyme
[0032] Weigh 50 mg of coenzyme NAD and add it to 10 ml of phosphate buffer (pH 7.0) containing 50 mg / ml carbonyl reductase, and let stand at 4°C for 1 h; add 1.5 g of SBA prepared in Example 1 to the above solution -15 / Fe 3 O 4 -NH 2 , 25℃, 100-150 rpm, incubate for 8 h, obtain the suspension, remove the supernatant by magnetic adsorption, wash the precipitate with physiological saline, repeat three times, the obtained solid is the co-immobilized enzyme of carbonyl reductase and coenzyme, freeze-dried Store at 4°C for later use.
Embodiment 3
[0033] Example 3 This example illustrates another method of co-immobilizing enzymes
[0034] Weigh 50 mg of coenzyme NADP and add it to 10 ml of phosphate buffer (pH 7.0) containing 50 mg / ml ketoreductase, and let stand at 4°C for 1 h; add 1.5 g of SBA prepared in Example 1 to the above solution -15 / Fe 3 O 4 -NH 2 , 25℃, 100-150 rpm, warm bath for 8 hours, obtain the suspension, remove the supernatant by magnetic adsorption, wash the precipitate with physiological saline, repeat three times, the obtained solid is the co-immobilized enzyme of carbonyl reductase and coenzyme, freeze-dried Store at 4°C for later use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com