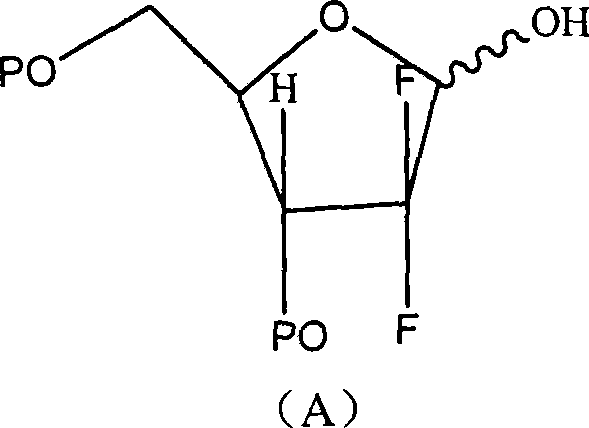
Method for preparing gemcitabine hydrochloride
A technology of gemcitabine hydrochloride and its synthesis method, which is applied in the field of synthesis of new anti-cancer drug gemcitabine hydrochloride, and can solve the problems of relatively expensive price and high cost of the protecting group TBS
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
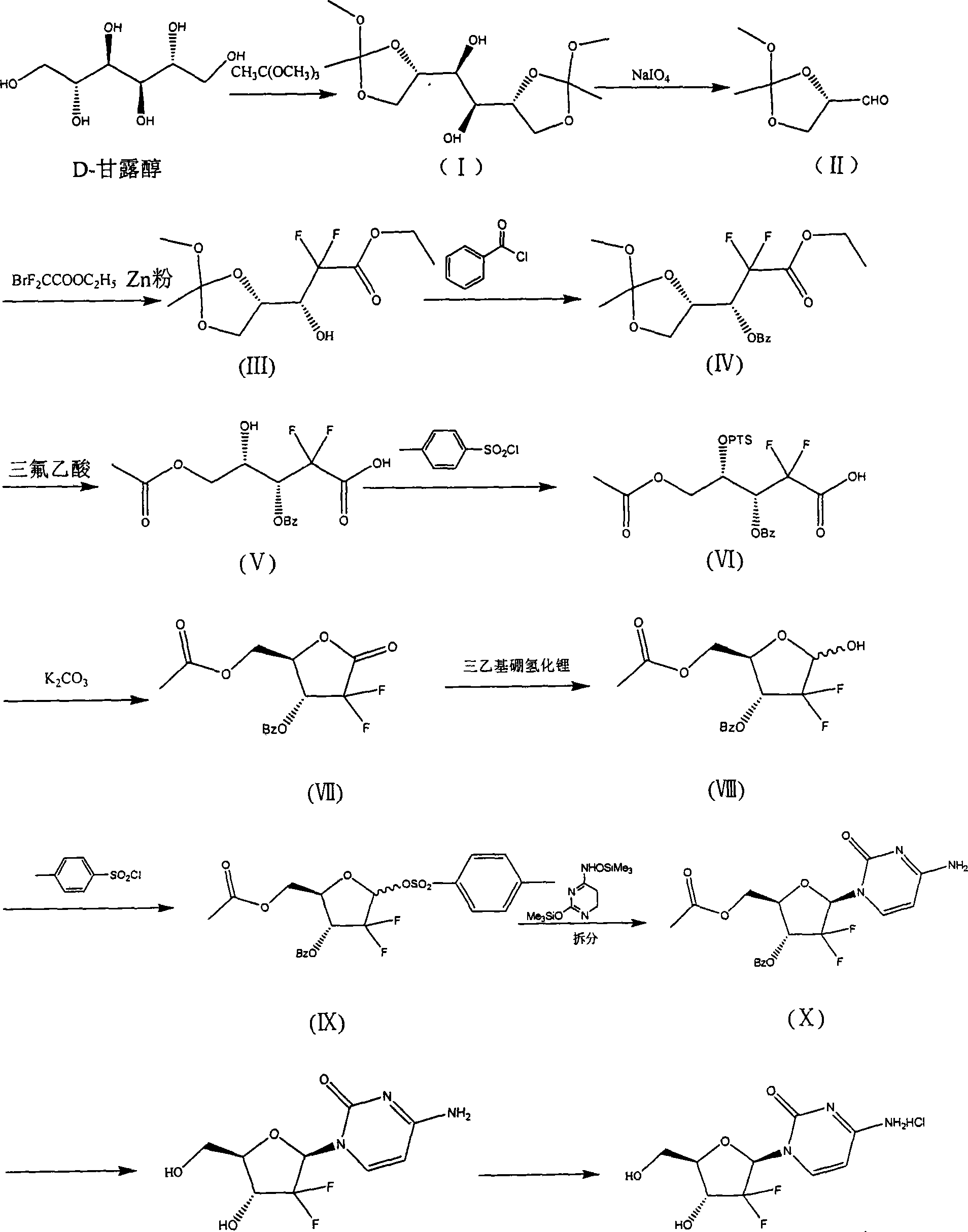
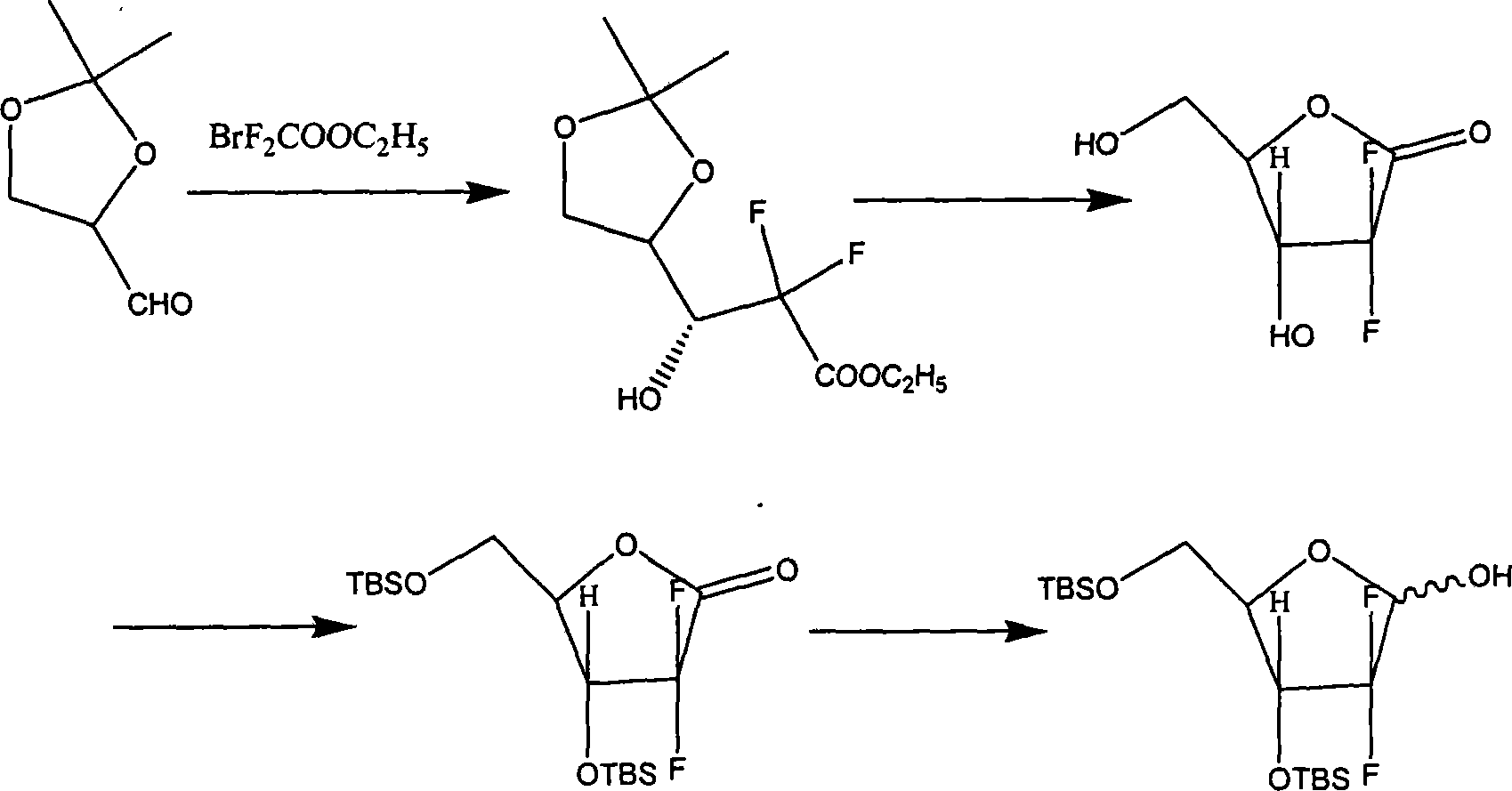
[0092] Example 1: (1S, 2S)-2-((4R)-2-methoxy-2-methyl-1,3-dioxocyclopentyl)-1-((4S)-2-methyl Synthesis of oxy-2-methyl-1,3-dioxocyclopentyl)-1,2-ethanediol
[0093] After dissolving 180 g of D-mannitol in 1000 ml of DMF, add 2 g of p-toluenesulfonic acid and 300 g of trimethyl orthoacetate, react at room temperature for 24 hours, and adjust the pH of the solution to 7.5 with 15% ammonia water. Filter, concentrate the filtrate to 100ml under reduced pressure, add 500ml of water, stir evenly, add 300ml*3 dichloromethane for extraction, combine the dichloromethane layer, add 50g of anhydrous sodium sulfate, stir for 2 hours, filter to obtain the target product dichloromethane solution.
Embodiment 2
[0094] Example 2: Synthesis of (4S)-2-methoxy-2-methyl-1,3-dioxocyclopentyl-4-aldehyde
[0095] In the dichloromethane solution obtained in Example 1, 700ml aqueous solution containing 180g of sodium periodate was added in portions, stirred at room temperature for 4 hours, filtered, and the dichloromethane layer was collected by standing, dried over anhydrous sodium sulfate, and then heated at 30°C Concentrate under reduced pressure until no liquid flows out of the condenser. Control the external temperature at 120°C to collect distillates at 30-100°C under reduced pressure, combine the distillates, add 50 g of anhydrous sodium sulfate, stir for 2 hours, filter, and concentrate the filtrate below 30°C to dryness under reduced pressure to obtain 95-100 g of the target product.
Embodiment 3
[0096] Example 3: (R)-2,2-difluoro-3-hydroxyl-3-((4S)-2-methoxy-2-methyl-1,3-dioxocyclopentyl)propionic acid Synthesis of Ethyl Ester
[0097] In 200ml tetrahydrofuran, add 45g of activated zinc powder, control the internal temperature of 45-50°C and add dropwise the product obtained in Example 2 and 104g ethyl difluorobromoacetate mixture in 200ml tetrahydrofuran solution, drop it in about 1 hour, and keep warm for 2 hours. TLC detected that the reaction was complete. After the reaction solution was lowered to around 0°C, it was poured into a mixture of 200g ice, 400g water and 72g concentrated hydrochloric acid, stirred for 30 minutes, filtered, extracted with 160ml*2 dichloromethane, 5% carbonic acid After the dichloromethane layer was washed with 200 ml of sodium hydrogen, it was washed once with 150 ml of saturated brine. The collected organic layers were dried over 50 g of anhydrous sodium sulfate, filtered, and concentrated to dryness in vacuo. Petroleum ether: ethyl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com