Preparation method of ursodeoxycholic acid
A technology for ursodeoxycholic acid and oxidation reaction, which is applied in the field of preparation of ursodeoxycholic acid, can solve the problems of high price of ursodeoxycholic acid and high requirements on preparation technology, and achieves low cost, stable quality and product purity. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
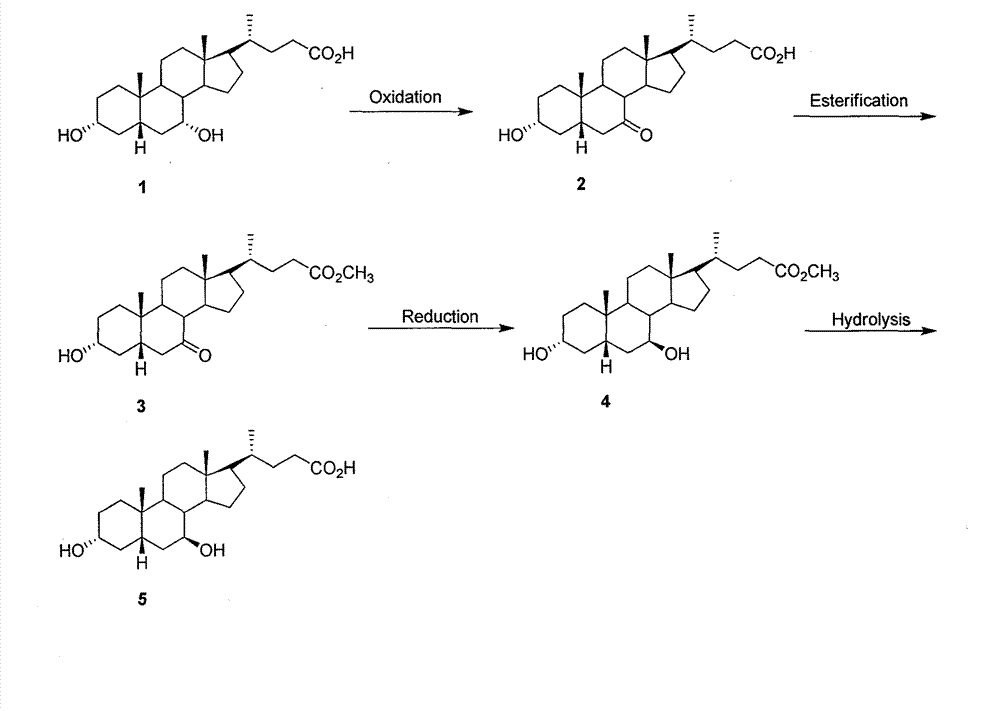
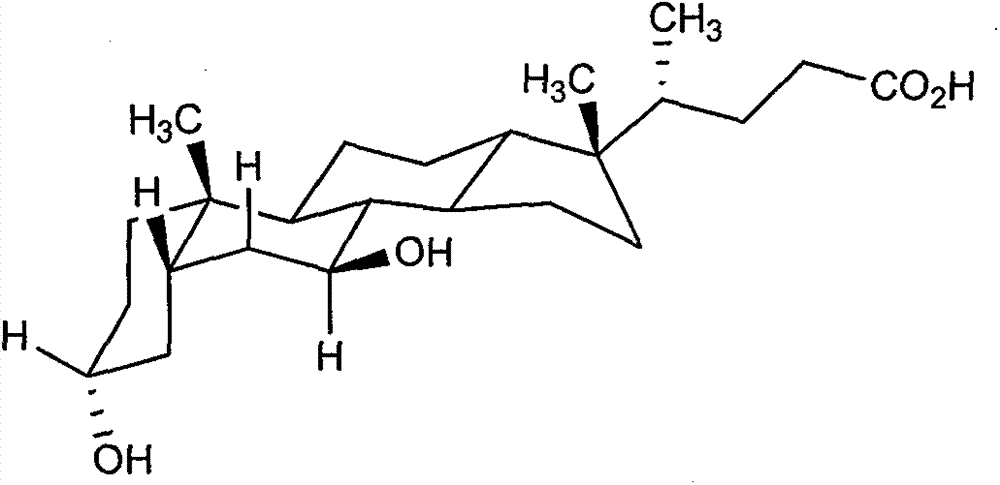
[0015] The preparation of ursodeoxycholic acid among the present invention is realized through the following steps:
[0016] The specific steps are:
[0017] (1) N-bromosuccinimide (NBS) was used to selectively oxidize the hydroxyl group at the C-7 position of chenodeoxycholic acid.
[0018] (2) Methyl monooxide was catalyzed by concentrated hydrochloric acid in methanol solution.
[0019] (3) Using NaBH 4 / CeCl 3 The system reduces the carbonyl group to increase the α / β selectivity.
[0020] (4) The hydrolysis reaction is carried out in a sodium hydroxide methanol solution with a concentration of 1 mol / L.
[0021] The organic solvent described in the step (1) of the present invention is a mixed solvent of acetone and water, the molar ratio of the oxidizing agent and chenodeoxycholic acid is optimally 1:1.6, the reaction temperature is 25° C., and the reaction time is 2 hours.
[0022] The organic solvent described in the step (2) of the present invention is methanol, whi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com