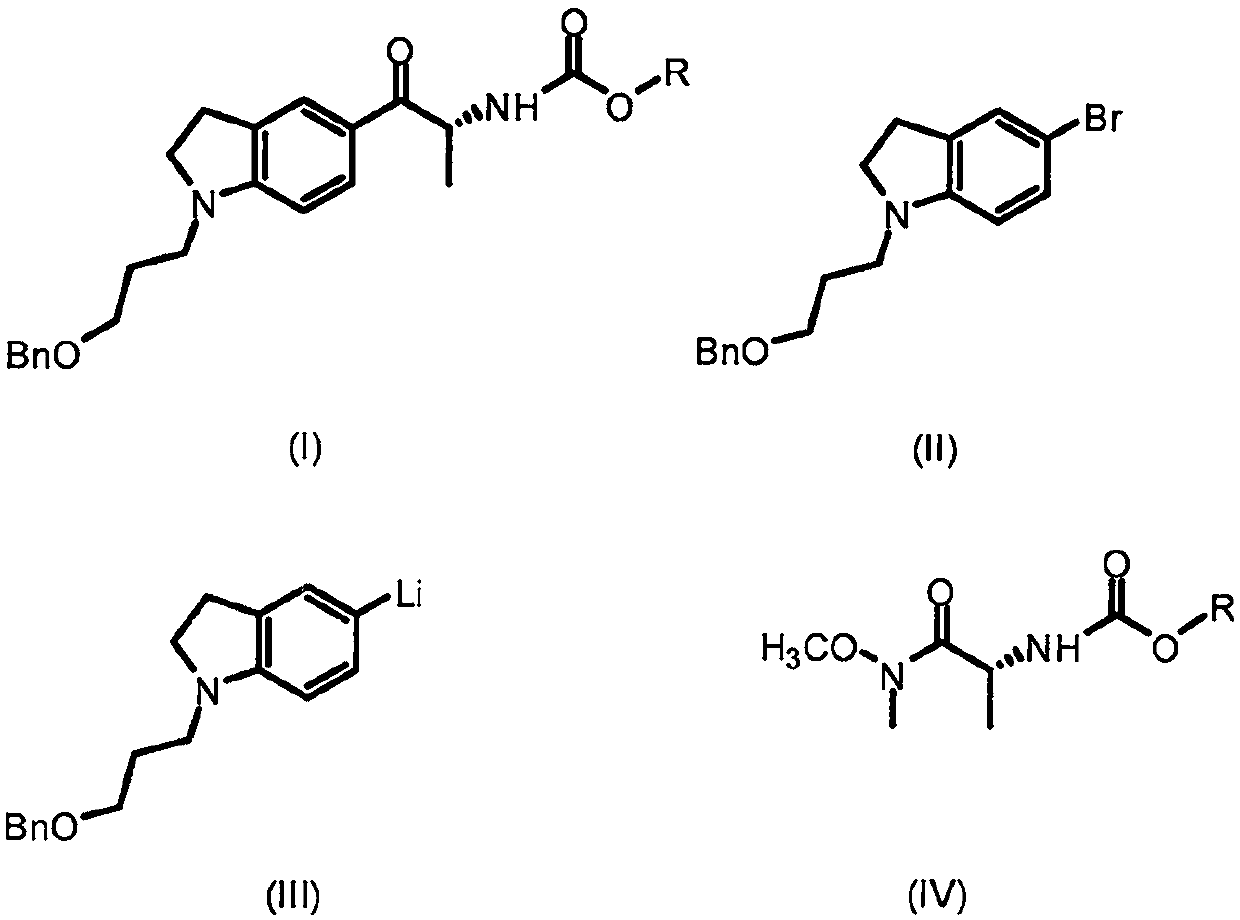
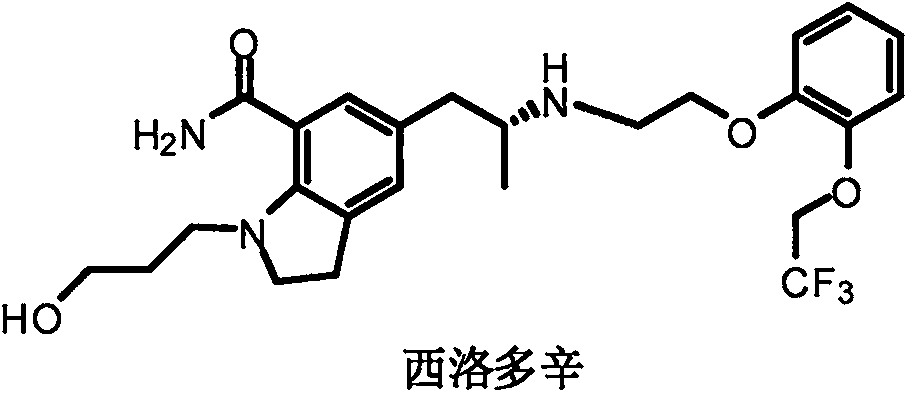
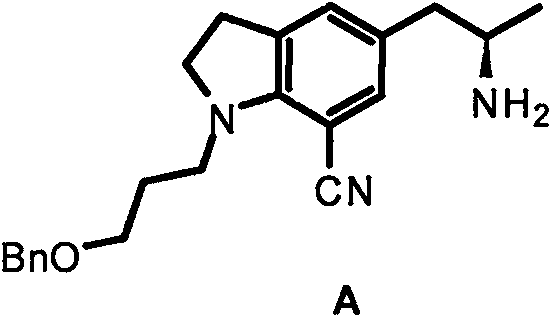
Method for preparing silodosin chiral intermediate
A technology of intermediates and synthetic methods, applied in the field of drug synthesis, can solve the problems of chiral resolution waste, expensive raw materials, and low yield of follow-up reactions, etc., and achieve the effects of low product cost, simple reaction operation, and high application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]
[0026] Take a three-necked reaction flask to vacuum and then fill it with nitrogen, repeat three times, add 34.6g (100mmol) of 1-[3-(benzyloxy)propyl]-5-bromoindoline and 200mL tetrahydrofuran. At -78°C, 50 mL (2.4 M, 120 mmol) of a n-butyllithium solution in n-hexane was slowly added dropwise, and stirring was continued for 15 minutes after the addition was complete. Take 31.9 g (120 mmol) of (R)-N-benzyloxycarbonyl-alanine Weinreb amide, dissolve it in 200 mL of tetrahydrofuran, and slowly add it dropwise to the above reaction solution at -78°C. Slowly warm to room temperature and stir overnight. Slowly add 400 mL of saturated ammonium chloride solution under ice bath, stir for 30 minutes, and transfer to a separatory funnel. Extracted with ethyl acetate (300 mL×3), the combined organic layer was washed twice with 400 mL saturated brine, and then dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure, and the residue was purified by...
Embodiment 2
[0028] Using the same procedure as in Example 1, 92.3 mL of sec-butyl lithium (120 mmol, 1.3 M cyclohexane solution) was used instead of n-butyl lithium to obtain 30.0 g of the product with a yield of 63.6% and an ee value of 99.9%.
Embodiment 3
[0030] Using the same procedure as in Example 1, 92.3mL of tert-butyllithium (120mmol, 1.3M pentane solution) was used instead of n-butyllithium to obtain 26.6g of the product with a yield of 56.3% and an ee value of 99.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com