Preparation method of silodosin intermediate
A technology of silodosin and intermediates, applied in the field of pharmaceutical synthesis, can solve the problems of compound IX yield reduction, impact on compound XII yield, low selectivity of hydroformylation reaction, etc., and achieve the effect of improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
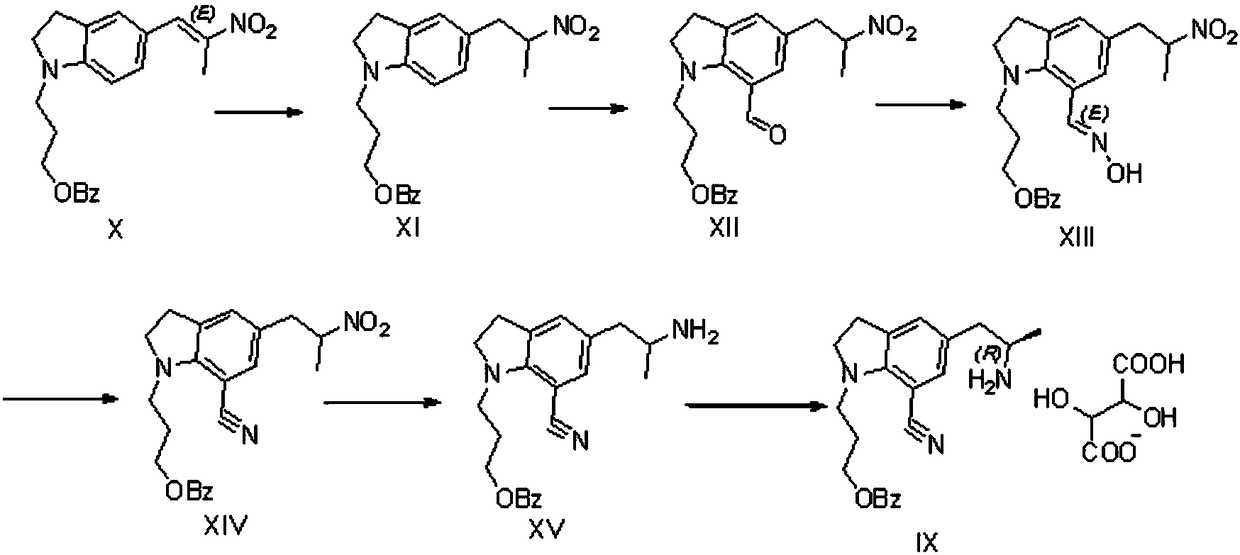
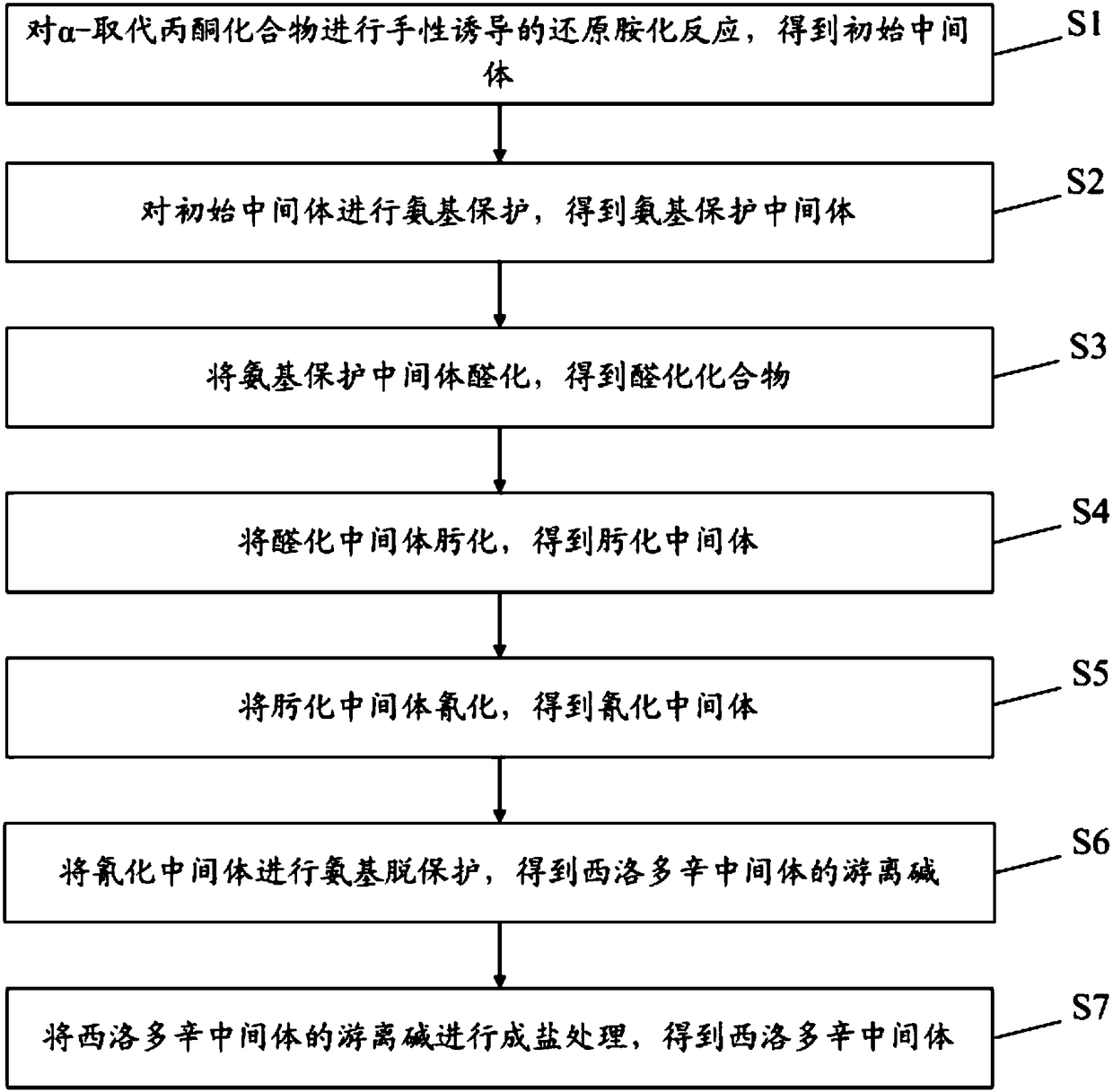
[0055] see figure 2 , the preparation method of silodosin intermediate provided in the embodiment of the present invention comprises:
[0056] S1: chiral-induced reductive amination of α-substituted acetonides to obtain initial intermediates; wherein,
[0057] The structural formula of α-substituted acetonides is:
[0058]
[0059] The structural formula of the initial intermediate is:
[0060]
[0061] S2: carry out amino protection to the initial intermediate to obtain an amino-protected intermediate; wherein, the structural formula of the amino-protected intermediate is:
[0062]
[0063] S3: hydroformylation of the amino-protected intermediate to obtain an hydroformylation intermediate; wherein,
[0064] The structural formula of the hydroformylation intermediate is:
[0065]
[0066] S4: oximation of the hydroformylation intermediate to obtain the oximation intermediate; wherein,
[0067] The structural formula of the oximation intermediate is:
[0068]...
Embodiment 1
[0134] The present embodiment provides a kind of preparation method of silodosin intermediate, comprising:
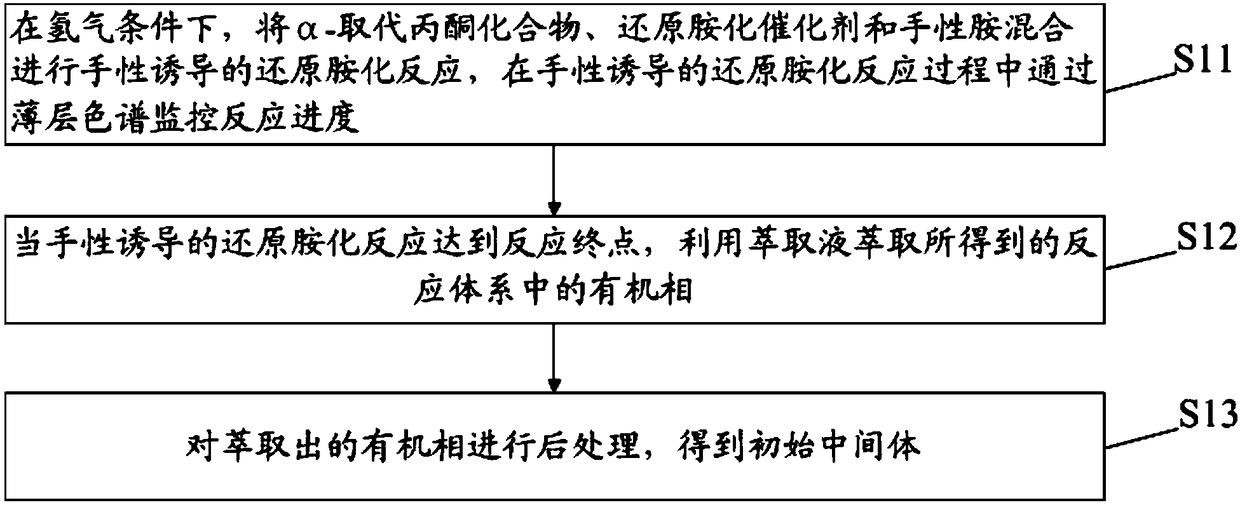
[0135] In the first step, the α-substituted acetonide was dissolved in tetrahydrofuran under hydrogen to make an initial solution, and then platinum oxide and R-phenylethylamine were added to it, and the above substances were stirred at room temperature for chiral-induced reduction Amination reaction, monitor the reductive amination reaction progress by thin-layer chromatography after 8 hours; Wherein, the mass ratio of α-substituted acetonide and platinum oxide is 1:0.1%, the molar ratio of α-substituted acetonide and R-phenylethylamine The ratio is 1:1.2;
[0136] When the reductive amination reaction reaches the end of the reaction, the platinum black obtained by the platinum oxidation reaction is recovered by filtration, the solvent tetrahydrofuran is recovered by rotary evaporation, and then ethyl acetate and hydrochloric acid are added to the residue to extract th...
Embodiment 2
[0162] The present embodiment provides a preparation method of a silodosin intermediate, comprising:
[0163] In the first step, the α-substituted acetone compound was dissolved in tetrahydrofuran to make the initial solution, then R-naphthaleneethylamine and sodium borohydride were added to it, and the above substances were stirred at room temperature to carry out reductive amination reaction, after 8 hours The progress of the reductive amination reaction was monitored by thin-layer chromatography; wherein, the molar ratio of α-substituted acetone to sodium borohydride was 1:1.80, and the molar ratio of α-substituted acetone to R-naphthylethylamine was 1:1.12;
[0164] When the reductive amination reaction reaches the reaction end point, the platinum black obtained by the platinum oxide reaction is filtered and recovered, the solvent tetrahydrofuran is recovered by rotary evaporation, and then ethyl acetate and hydrochloric acid are sequentially added to the residue to extract...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com